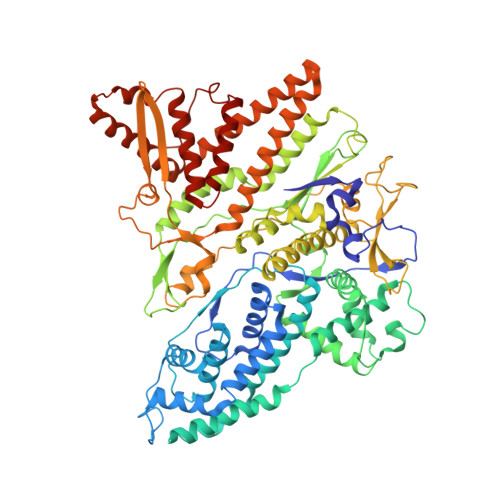
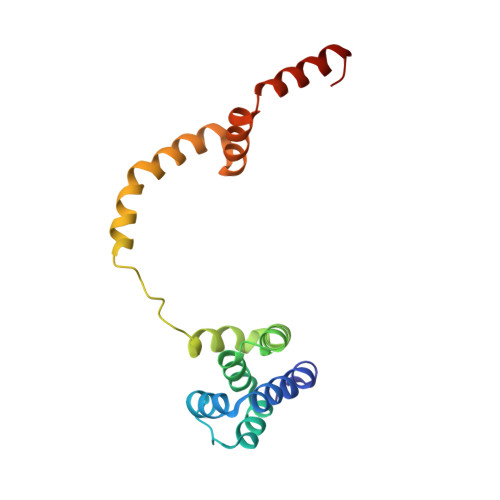
Double-stranded RNA virus outer shell assembly by bona fide domain-swapping.
Sun, Z., El Omari, K., Sun, X., Ilca, S.L., Kotecha, A., Stuart, D.I., Poranen, M.M., Huiskonen, J.T.(2017) Nat Commun 8: 14814-14814
- PubMed: 28287099
- DOI: https://doi.org/10.1038/ncomms14814
- Primary Citation of Related Structures:
5MUU, 5MUV, 5MUW - PubMed Abstract:
Correct outer protein shell assembly is a prerequisite for virion infectivity in many multi-shelled dsRNA viruses. In the prototypic dsRNA bacteriophage φ6, the assembly reaction is promoted by calcium ions but its biomechanics remain poorly understood. Here, we describe the near-atomic resolution structure of the φ6 double-shelled particle. The outer T=13 shell protein P8 consists of two alpha-helical domains joined by a linker, which allows the trimer to adopt either a closed or an open conformation. The trimers in an open conformation swap domains with each other. Our observations allow us to propose a mechanistic model for calcium concentration regulated outer shell assembly. Furthermore, the structure provides a prime exemplar of bona fide domain-swapping. This leads us to extend the theory of domain-swapping from the level of monomeric subunits and multimers to closed spherical shells, and to hypothesize a mechanism by which closed protein shells may arise in evolution.
Organizational Affiliation:
Division of Structural Biology, Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford OX3 7BN, UK.