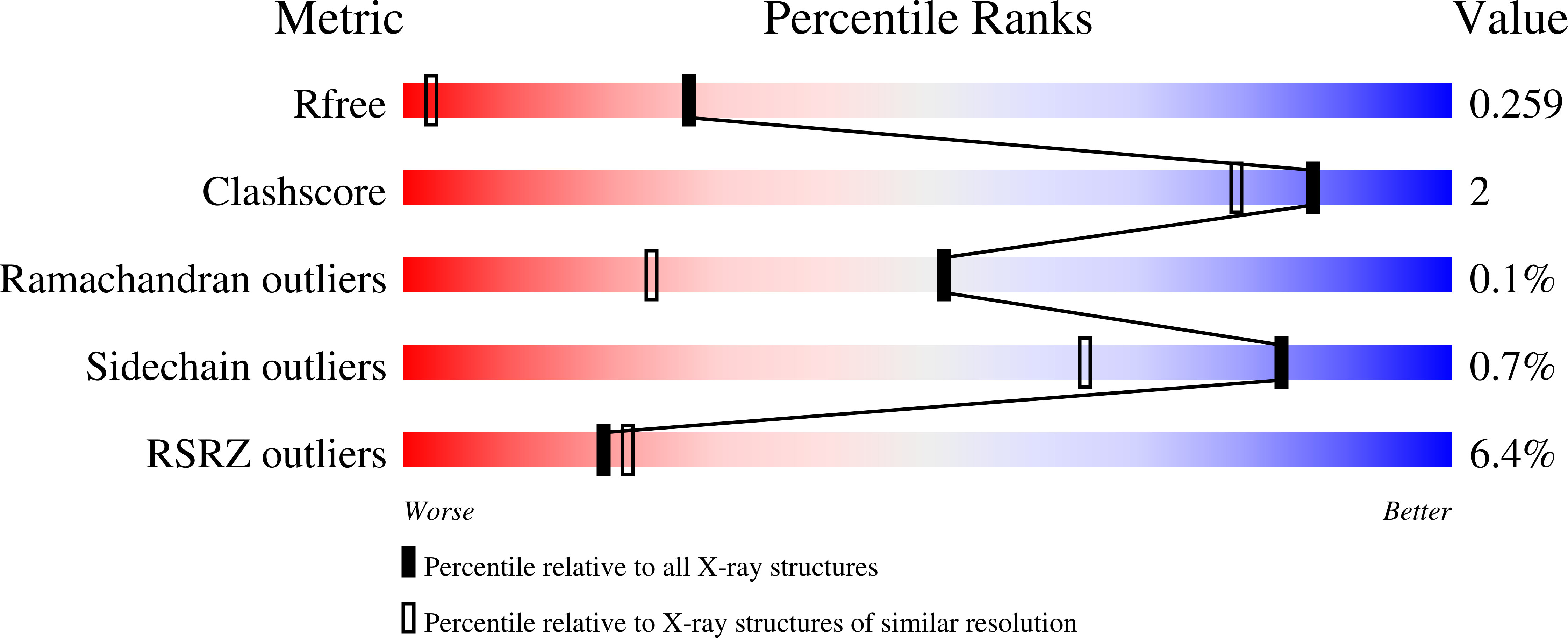
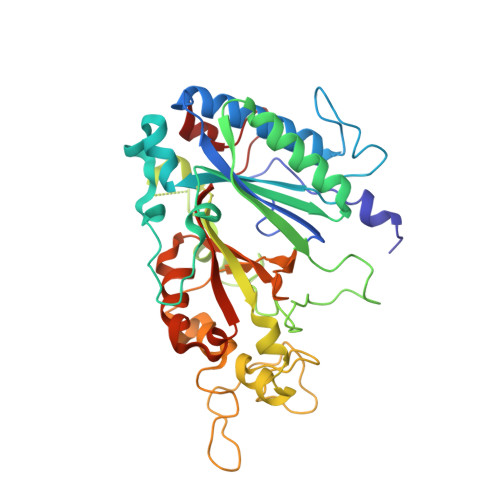
Photoreduction and validation of haem-ligand intermediate states in protein crystals by in situ single-crystal spectroscopy and diffraction.
Kekilli, D., Moreno-Chicano, T., Chaplin, A.K., Horrell, S., Dworkowski, F.S.N., Worrall, J.A.R., Strange, R.W., Hough, M.A.(2017) IUCrJ 4: 263-270
- PubMed: 28512573
- DOI: https://doi.org/10.1107/S2052252517002159
- Primary Citation of Related Structures:
5MAP, 5MJH - PubMed Abstract:
Powerful synergies are available from the combination of multiple methods to study proteins in the crystalline form. Spectroscopies which probe the same region of the crystal from which X-ray crystal structures are determined can give insights into redox, ligand and spin states to complement the information gained from the electron-density maps. The correct assignment of crystal structures to the correct protein redox and ligand states is essential to avoid the misinterpretation of structural data. This is a particular concern for haem proteins, which can occupy a wide range of redox states and are exquisitely sensitive to becoming reduced by solvated electrons generated from interactions of X-rays with water molecules in the crystal. Here, single-crystal spectroscopic fingerprinting has been applied to investigate the laser photoreduction of ferric haem in cytochrome c '. Furthermore, in situ X-ray-driven generation of haem intermediates in crystals of the dye-decolourizing-type peroxidase A (DtpA) from Streptomyces lividans is described.
Organizational Affiliation:
School of Biological Sciences, University of Essex, Wivenhoe Park, Colchester CO4 3SQ, England.