RavN is a member of a previously unrecognized group of Legionella pneumophila E3 ubiquitin ligases.
Lin, Y.H., Lucas, M., Evans, T.R., Abascal-Palacios, G., Doms, A.G., Beauchene, N.A., Rojas, A.L., Hierro, A., Machner, M.P.(2018) PLoS Pathog 14: e1006897-e1006897
- PubMed: 29415051
- DOI: https://doi.org/10.1371/journal.ppat.1006897
- Primary Citation of Related Structures:
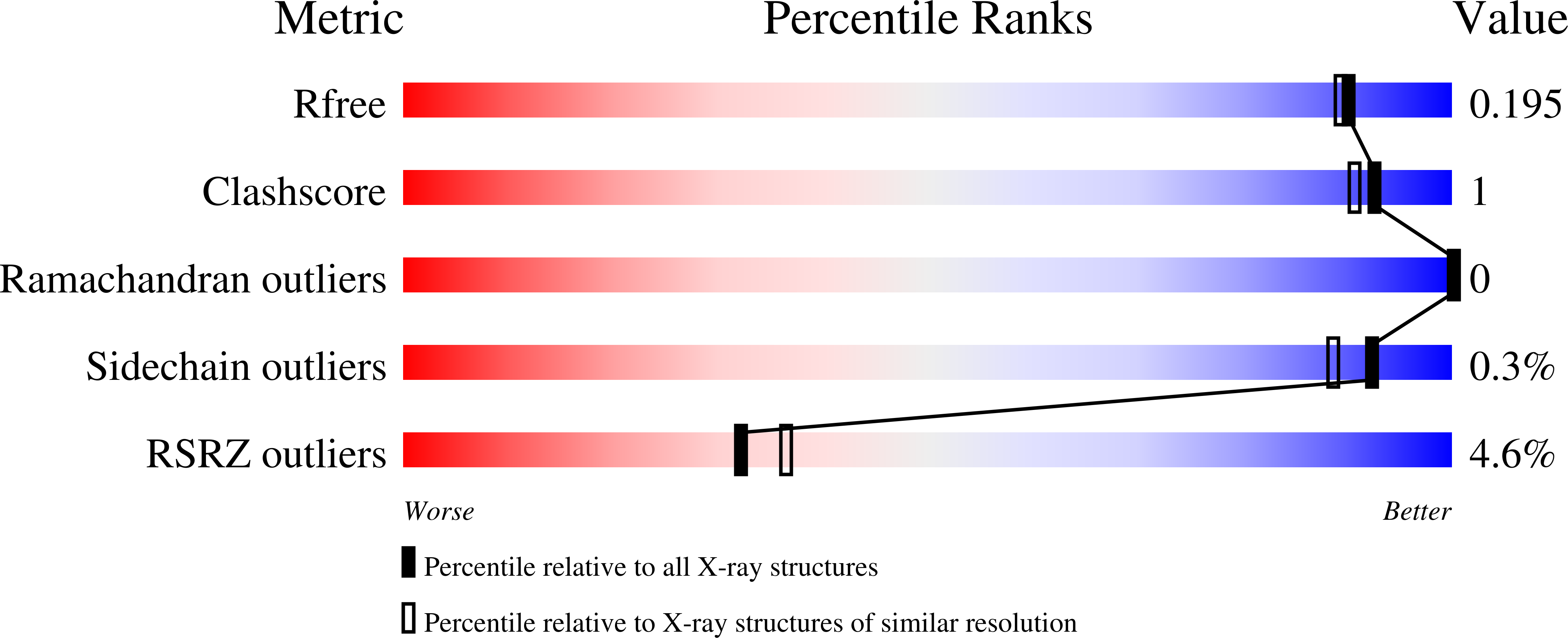
5MIY - PubMed Abstract:
The eukaryotic ubiquitylation machinery catalyzes the covalent attachment of the small protein modifier ubiquitin to cellular target proteins in order to alter their fate. Microbial pathogens exploit this post-translational modification process by encoding molecular mimics of E3 ubiquitin ligases, eukaryotic enzymes that catalyze the final step in the ubiquitylation cascade. Here, we show that the Legionella pneumophila effector protein RavN belongs to a growing class of bacterial proteins that mimic host cell E3 ligases to exploit the ubiquitylation pathway. The E3 ligase activity of RavN was located within its N-terminal region and was dependent upon interaction with a defined subset of E2 ubiquitin-conjugating enzymes. The crystal structure of the N-terminal region of RavN revealed a U-box-like motif that was only remotely similar to other U-box domains, indicating that RavN is an E3 ligase relic that has undergone significant evolutionary alteration. Substitution of residues within the predicted E2 binding interface rendered RavN inactive, indicating that, despite significant structural changes, the mode of E2 recognition has remained conserved. Using hidden Markov model-based secondary structure analyses, we identified and experimentally validated four additional L. pneumophila effectors that were not previously recognized to possess E3 ligase activity, including Lpg2452/SdcB, a new paralog of SidC. Our study provides strong evidence that L. pneumophila is dedicating a considerable fraction of its effector arsenal to the manipulation of the host ubiquitylation pathway.
Organizational Affiliation:
Division of Molecular and Cellular Biology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, United States of America.