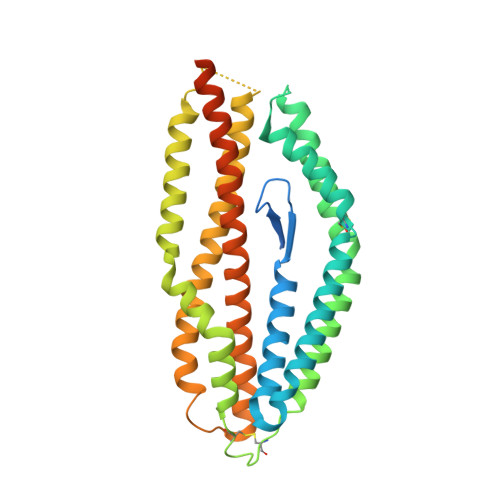
One-step design of a stable variant of the malaria invasion protein RH5 for use as a vaccine immunogen.
Campeotto, I., Goldenzweig, A., Davey, J., Barfod, L., Marshall, J.M., Silk, S.E., Wright, K.E., Draper, S.J., Higgins, M.K., Fleishman, S.J.(2017) Proc Natl Acad Sci U S A 114: 998-1002
- PubMed: 28096331
- DOI: https://doi.org/10.1073/pnas.1616903114
- Primary Citation of Related Structures:
5MI0 - PubMed Abstract:
Many promising vaccine candidates from pathogenic viruses, bacteria, and parasites are unstable and cannot be produced cheaply for clinical use. For instance, Plasmodium falciparum reticulocyte-binding protein homolog 5 (PfRH5) is essential for erythrocyte invasion, is highly conserved among field isolates, and elicits antibodies that neutralize in vitro and protect in an animal model, making it a leading malaria vaccine candidate. However, functional RH5 is only expressible in eukaryotic systems and exhibits moderate temperature tolerance, limiting its usefulness in hot and low-income countries where malaria prevails. Current approaches to immunogen stabilization involve iterative application of rational or semirational design, random mutagenesis, and biochemical characterization. Typically, each round of optimization yields minor improvement in stability, and multiple rounds are required. In contrast, we developed a one-step design strategy using phylogenetic analysis and Rosetta atomistic calculations to design PfRH5 variants with improved packing and surface polarity. To demonstrate the robustness of this approach, we tested three PfRH5 designs, all of which showed improved stability relative to wild type. The best, bearing 18 mutations relative to PfRH5, expressed in a folded form in bacteria at >1 mg of protein per L of culture, and had 10-15 °C higher thermal tolerance than wild type, while also retaining ligand binding and immunogenic properties indistinguishable from wild type, proving its value as an immunogen for a future generation of vaccines against the malaria blood stage. We envision that this efficient computational stability design methodology will also be used to enhance the biophysical properties of other recalcitrant vaccine candidates from emerging pathogens.
Organizational Affiliation:
Department of Biochemistry, University of Oxford, Oxford OX1 3QU, United Kingdom.