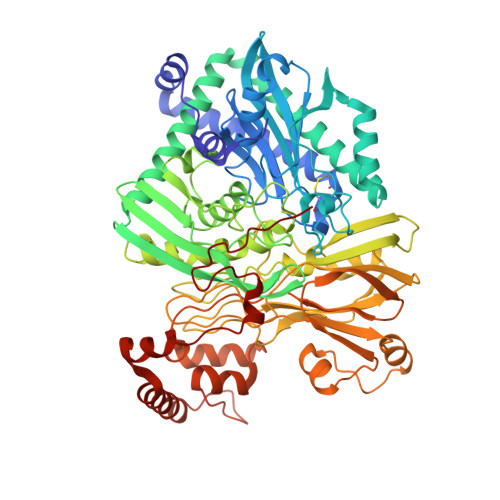
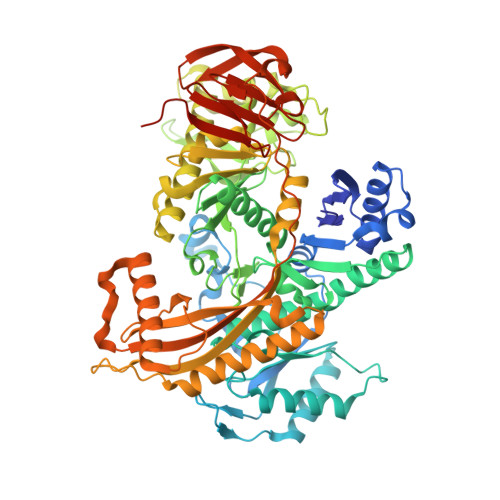
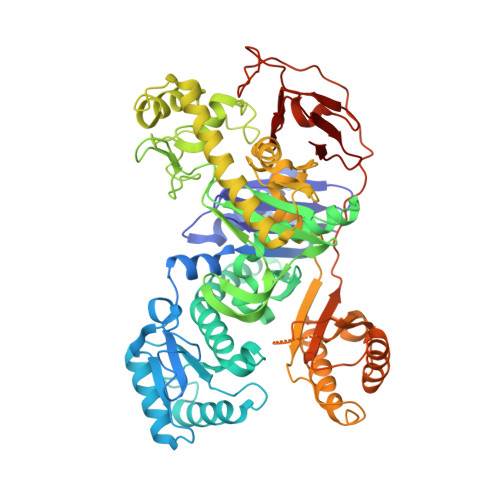
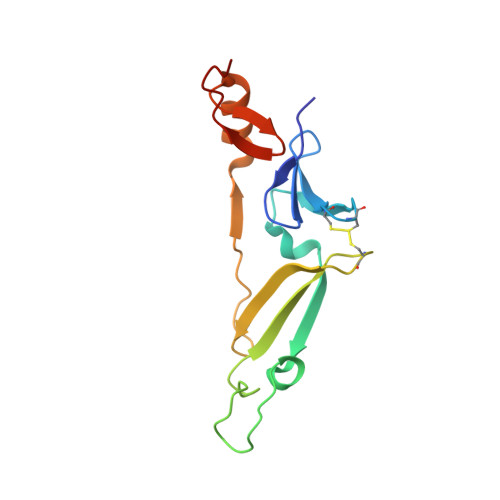
Structure of the acetophenone carboxylase core complex: prototype of a new class of ATP-dependent carboxylases/hydrolases.
Weidenweber, S., Schuhle, K., Demmer, U., Warkentin, E., Ermler, U., Heider, J.(2017) Sci Rep 7: 39674-39674
- PubMed: 28054554
- DOI: https://doi.org/10.1038/srep39674
- Primary Citation of Related Structures:
5L9W - PubMed Abstract:
Degradation of the aromatic ketone acetophenone is initiated by its carboxylation to benzoylacetate catalyzed by acetophenone carboxylase (Apc) in a reaction dependent on the hydrolysis of two ATP to ADP and P i . Apc is a large protein complex which dissociates during purification into a heterooctameric Apc(αα'βγ) 2 core complex of 482 kDa and Apcε of 34 kDa. In this report, we present the X-ray structure of the Apc(αα'βγ) 2 core complex from Aromatoleum aromaticum at ca. 3 Å resolution which reveals a unique modular architecture and serves as model of a new enzyme family. Apcβ contains a novel domain fold composed of two β-sheets in a barrel-like arrangement running into a bundle of eight short polyproline (type II)-like helical segments. Apcα and Apcα' possess ATP binding modules of the ASKHA superfamily integrated into their multidomain structures and presumably operate as ATP-dependent kinases for acetophenone and bicarbonate, respectively. Mechanistic aspects of the novel carboxylation reaction requiring massive structural rearrangements are discussed and criteria for specifically annotating the family members Apc, acetone carboxylase and hydantoinase are defined.
Organizational Affiliation:
Max-Planck-Institut für Biophysik, Max-von-Laue-Str. 3, 60438, Frankfurt am Main, Germany.