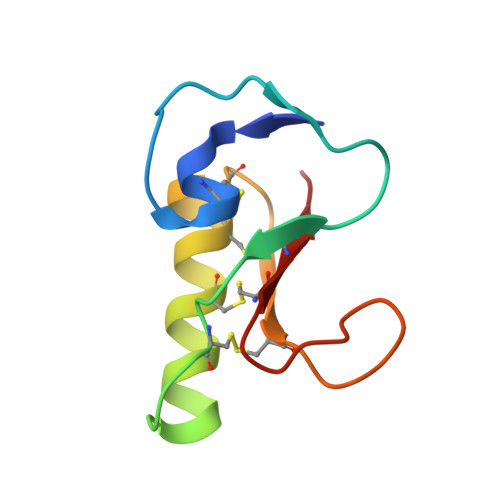
Structure and Function of FS50, a salivary protein from the flea Xenopsylla cheopis that blocks the sodium channel NaV1.5.
Xu, X., Zhang, B., Yang, S., An, S., Ribeiro, J.M., Andersen, J.F.(2016) Sci Rep 6: 36574-36574
- PubMed: 27819327
- DOI: https://doi.org/10.1038/srep36574
- Primary Citation of Related Structures:
5K6D - PubMed Abstract:
Naturally occurring toxins have been invaluable tools for the study of structural and functional relationships of voltage-gated sodium channels (VGSC). Few studies have been made of potential channel-modulating substances from blood-feeding arthropods. He we describe the characterization FS50, a salivary protein from the flea, Xenopsylla cheopis, that exhibits an inhibitory activity against the Na V 1.5 channel with an IC 50 of 1.58 μM. The pore-blocking mechanism of this toxin is evident from the kinetics of activation and inactivation suggesting that FS50 does not interfere with the voltage sensor of Na V 1.5. FS50 exhibits high specificity for Na V 1.5, since 10 μM FS50 had no discernable effect on voltage-gated Na + , K + and Ca 2+ channels in rat dorsal root ganglia or VGSC forms individually expressed in HEK 293T cells. Furthermore, intravenous injection of FS50 into rats and monkeys elicited recovery from arrhythmia induced by BaCl 2 , as would be expected from a blockade of Na V 1.5. The crystal structure of FS50 revealed a βαββ domain similar to that of scorpion β toxin and a small N-terminal βαβ domain. Site-directed mutagenesis experiments have implicated a basic surface including the side chains of Arg 6, His 11 and Lys 32 as potentially important in the FS50 Na V 1.5 interaction.
Organizational Affiliation:
Guangdong Provincial Key Laboratory of New Drug Screening, School of Pharmaceutical Sciences, Southern Medical University, Guangzhou 510515, Guangdong, China.