Structure of a pentameric virion-associated fiber with a potential role in Orsay virus entry to host cells.
Fan, Y., Guo, Y.R., Yuan, W., Zhou, Y., Holt, M.V., Wang, T., Demeler, B., Young, N.L., Zhong, W., Tao, Y.J.(2017) PLoS Pathog 13: e1006231-e1006231
- PubMed: 28241071
- DOI: https://doi.org/10.1371/journal.ppat.1006231
- Primary Citation of Related Structures:
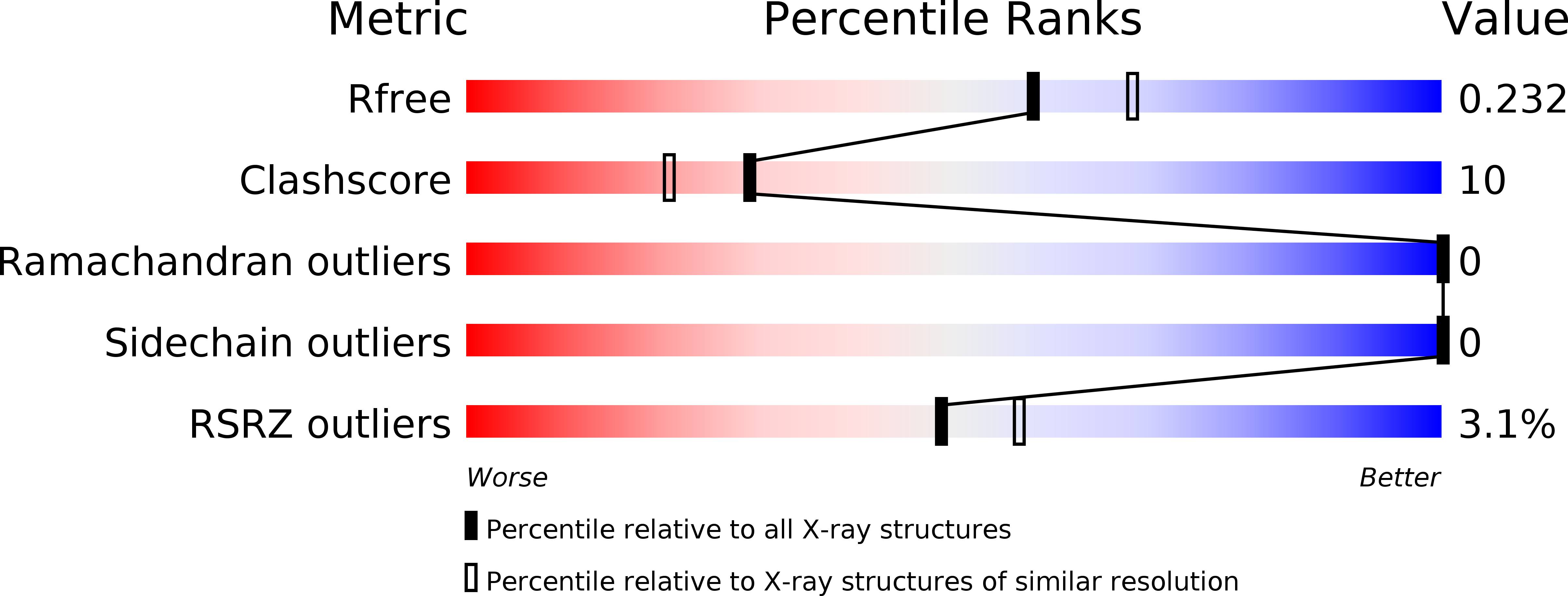
5JIE - PubMed Abstract:
Despite the wide use of Caenorhabditis elegans as a model organism, the first virus naturally infecting this organism was not discovered until six years ago. The Orsay virus and its related nematode viruses have a positive-sense RNA genome, encoding three proteins: CP, RdRP, and a novel δ protein that shares no homology with any other proteins. δ can be expressed either as a free δ or a CP-δ fusion protein by ribosomal frameshift, but the structure and function of both δ and CP-δ remain unknown. Using a combination of electron microscopy, X-ray crystallography, computational and biophysical analyses, here we show that the Orsay δ protein forms a ~420-Å long, pentameric fiber with an N-terminal α-helical bundle, a β-stranded filament in the middle, and a C-terminal head domain. The pentameric nature of the δ fiber has been independently confirmed by both mass spectrometry and analytical ultracentrifugation. Recombinant Orsay capsid containing CP-δ shows protruding long fibers with globular heads at the distal end. Mutant viruses with disrupted CP-δ fibers were generated by organism-based reverse genetics. These viruses were found to be either non-viable or with poor infectivity according to phenotypic and qRT-PCR analyses. Furthermore, addition of purified δ proteins to worm culture greatly reduced Orsay infectivity in a sequence-specific manner. Based on the structure resemblance between the Orsay CP-δ fiber and the fibers from reovirus and adenovirus, we propose that CP-δ functions as a cell attachment protein to mediate Orsay entry into worm intestine cells.
Organizational Affiliation:
Department of BioSciences, Rice University, MS-140, Houston, Texas, United States of America.