NMR Fragment Screening Hit Induces Plasticity of BRD7/9 Bromodomains
Wang, N., Li, F., Bao, H., Li, J., Wu, J., Ruan, K.(2016) Chembiochem 17: 1456-1463
- PubMed: 27194508
- DOI: https://doi.org/10.1002/cbic.201600184
- Primary Citation of Related Structures:
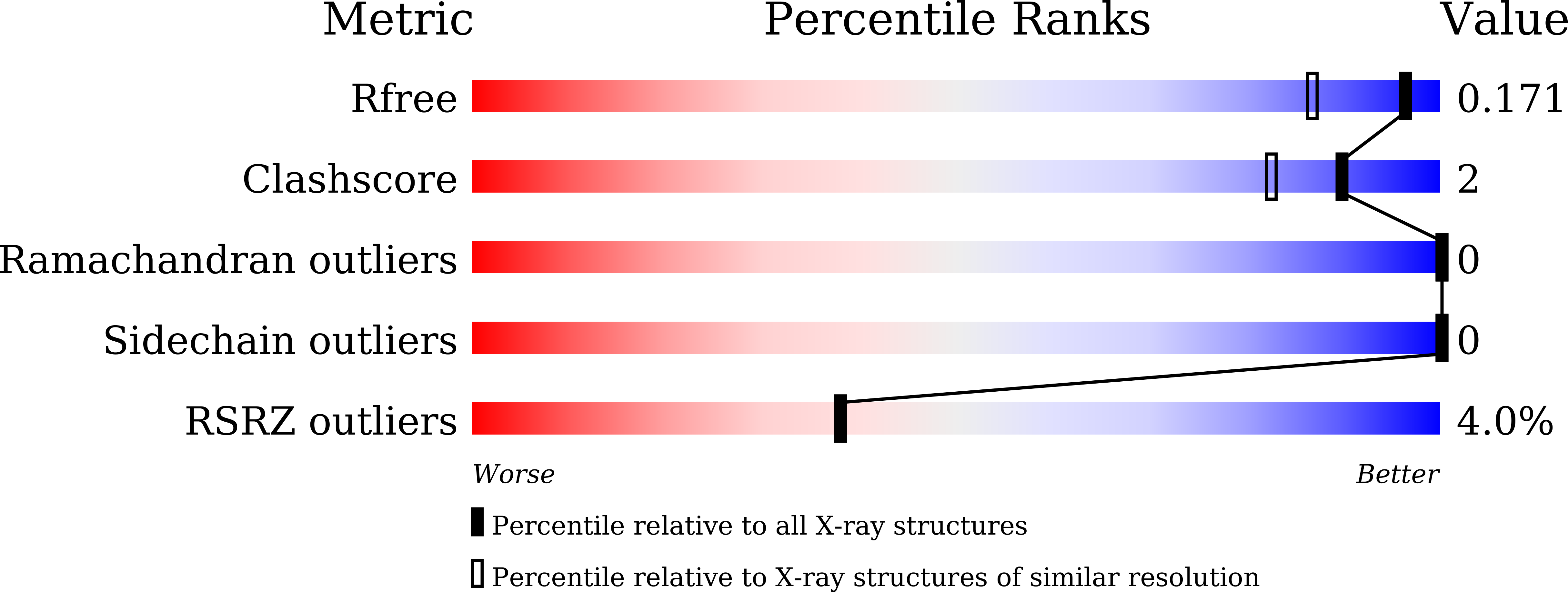
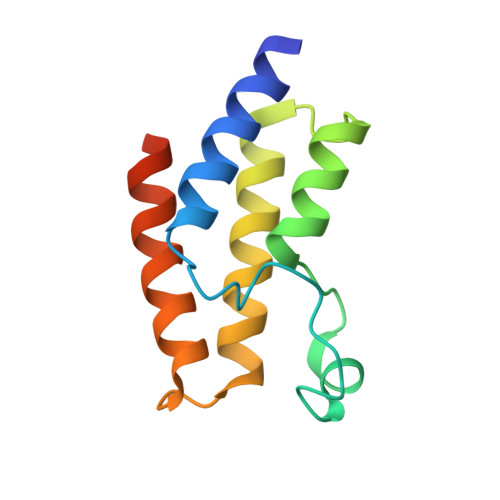
5JI8 - PubMed Abstract:
The complex biology associated with inhibition of bromodomain and extra-terminal (BET) domains by chemical probes has attracted increasing attention, and there is a need to identify non-BET bromodomain (BD) inhibitors. Several potent inhibitors of the BRD9 BD have recently been discovered, with anticancer and anti-inflammation activity. However, its paralogue, BRD7 BD, remains unexploited. Here, we identified new chemotypes targeting BRD7 BD by using NMR fragment-based screening. BRD7/9 BDs exhibit similar patterns of chemical-shift perturbation upon the titration of hit compound 1. The crystal structure revealed that 1 repels the Y222 group of BRD9 BD in a similar way to that for butyryllysine, but not acetyllysine and known inhibitors. Hit 1 induced less rearrangement of residue F161 of BRD9 BD than acetyllysine, butyryllysine, and crotonyllysine. Our study provides structural insight into a new generation of butyryllysine mimics for probing the function of BRD7/9 BD.
Organizational Affiliation:
Hefei National Laboratory for Physical Science at the Microscale, School of Life Sciences, University of Science and Technology of China, Hefei, Anhui, 230027, China.