Structural characterization of geranylgeranyl pyrophosphate synthase GACE1337 from the hyperthermophilic archaeon Geoglobus acetivorans.
Petrova, T.E., Boyko, K.M., Nikolaeva, A.Y., Stekhanova, T.N., Gruzdev, E.V., Mardanov, A.V., Stroilov, V.S., Littlechild, J.A., Popov, V.O., Bezsudnova, E.Y.(2018) Extremophiles 22: 877-888
- PubMed: 30062607
- DOI: https://doi.org/10.1007/s00792-018-1044-5
- Primary Citation of Related Structures:
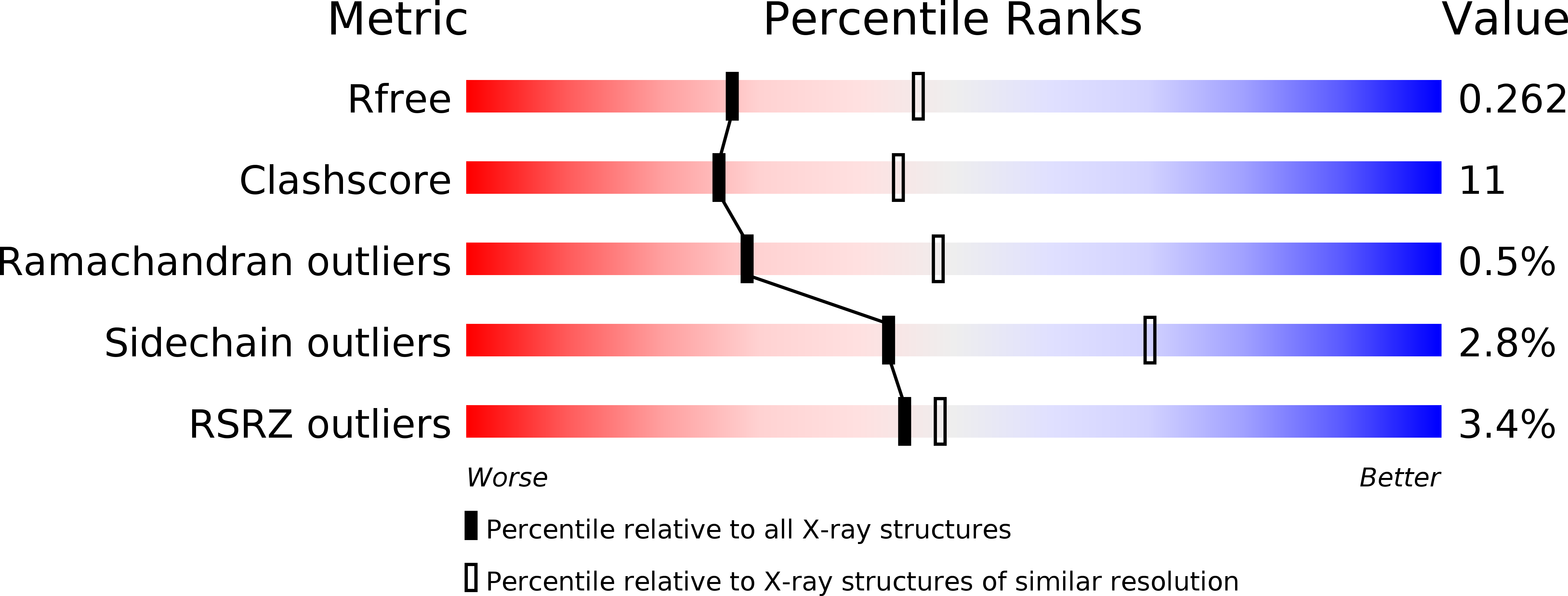
5JFQ - PubMed Abstract:
A novel type 1 geranylgeranyl pyrophosphate synthase GACE1337 has been identified within the genome of a newly identified hyperthermophilic archaeon Geoglobus acetivorans. The enzyme has been cloned and over-expressed in Escherichia coli. The recombinant enzyme has been biochemically and structurally characterized. It is able to catalyze the synthesis of geranylgeranyl pyrophosphate as a major product and of farnesyl pyrophosphate in smaller amounts, as measured by gas chromatography-mass spectrometry at an elevated temperature of 60 °C. Its ability to produce two products is consistent with the fact that GACE1337 is the only short-chain isoprenyl diphosphate synthase in G. acetivorans. Attempts to crystallize the enzyme were successful only at 37 °C. The three-dimensional structure of GACE1337 was determined by X-ray diffraction to 2.5 Å resolution. A comparison of its structure with those of related enzymes revealed that the Geoglobus enzyme has the features of both type I and type III geranylgeranyl pyrophosphate synthases, which allow it to regulate the product length. The active enzyme is a dimer and has three aromatic amino acids, two Phe, and a Tyr, located in the hydrophobic cleft between the two subunits. It is proposed that these bulky residues play a major role in the synthetic reaction by controlling the product elongation.
Organizational Affiliation:
Institute of Mathematical Problems of Biology, RAS, Branch of Keldysh Institute of Applied Mathematics of the Russian Academy of Sciences, Professor Vitkevich St., Pushchino, 142290, Russian Federation. tania.petrova.ru@gmail.com.