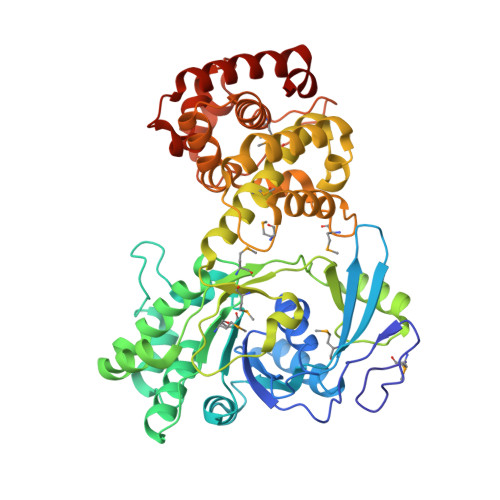
A highly efficient sorbitol dehydrogenase from Gluconobacter oxydans G624 and improvement of its stability through immobilization
Kim, T.S., Patel, S.K., Selvaraj, C., Jung, W.S., Pan, C.H., Kang, Y.C., Lee, J.K.(2016) Sci Rep 6: 33438-33438
- PubMed: 27633501
- DOI: https://doi.org/10.1038/srep33438
- Primary Citation of Related Structures:
5ITG - PubMed Abstract:
A sorbitol dehydrogenase (GoSLDH) from Gluconobacter oxydans G624 (G. oxydans G624) was expressed in Escherichia coli BL21(DE3)-CodonPlus RIL. The complete 1455-bp codon-optimized gene was amplified, expressed, and thoroughly characterized for the first time. GoSLDH exhibited Km and kcat values of 38.9 mM and 3820 s(-1) toward L-sorbitol, respectively. The enzyme exhibited high preference for NADP(+) (vs. only 2.5% relative activity with NAD(+)). GoSLDH sequencing, structure analyses, and biochemical studies, suggested that it belongs to the NADP(+)-dependent polyol-specific long-chain sorbitol dehydrogenase family. GoSLDH is the first fully characterized SLDH to date, and it is distinguished from other L-sorbose-producing enzymes by its high activity and substrate specificity. Isothermal titration calorimetry showed that the protein binds more strongly to D-sorbitol than other L-sorbose-producing enzymes, and substrate docking analysis confirmed a higher turnover rate. The high oxidation potential of GoSLDH for D-sorbitol was confirmed by cyclovoltametric analysis. Further, stability of GoSLDH significantly improved (up to 13.6-fold) after cross-linking of immobilized enzyme on silica nanoparticles and retained 62.8% residual activity after 10 cycles of reuse. Therefore, immobilized GoSLDH may be useful for L-sorbose production from D-sorbitol.
Organizational Affiliation:
Department of Chemical Engineering, Konkuk University, Seoul 05029, Korea.