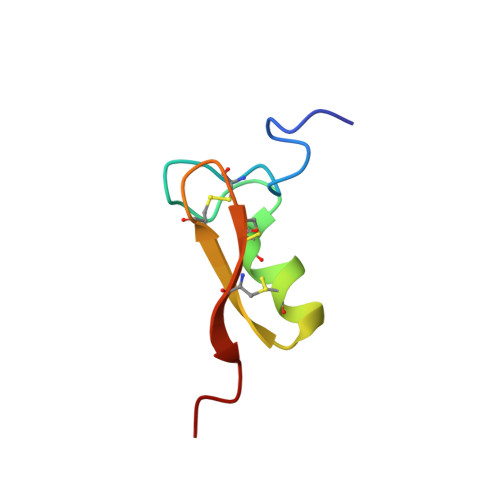
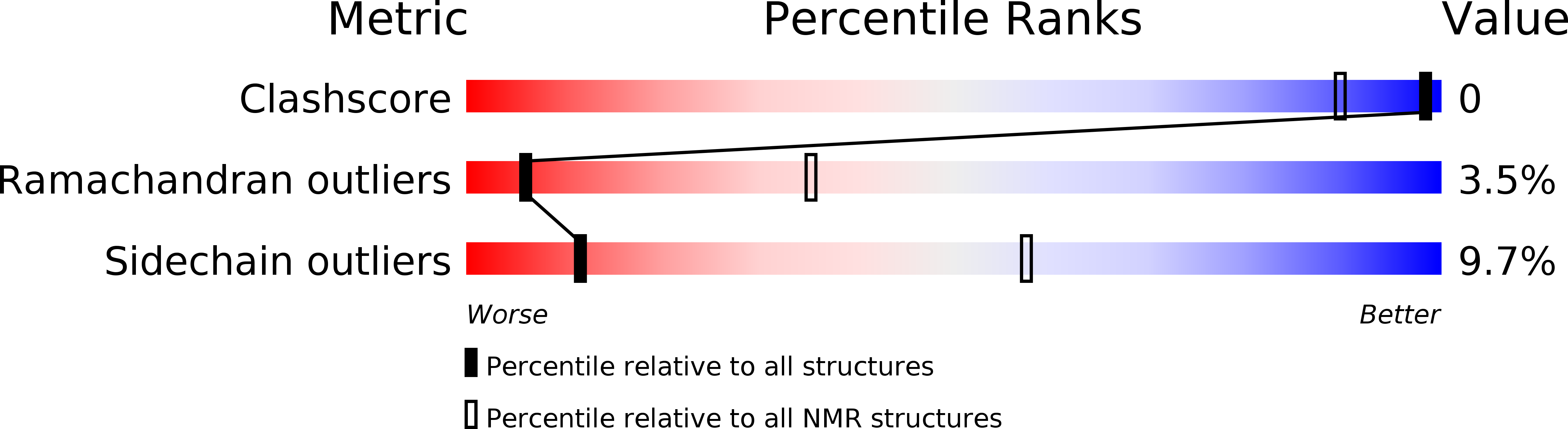Solution structure and antiparasitic activity of scorpine-like peptides from Hoffmannihadrurus gertschi.
Flores-Solis, D., Toledano, Y., Rodriguez-Lima, O., Cano-Sanchez, P., Ramirez-Cordero, B.E., Landa, A., Rodriguez de la Vega, R.C., Del Rio-Portilla, F.(2016) FEBS Lett 590: 2286-2296
- PubMed: 27314815
- DOI: https://doi.org/10.1002/1873-3468.12255
- Primary Citation of Related Structures:
5IPO, 5JYH - PubMed Abstract:
Scorpine-like peptides are two domain peptides found in different scorpion venoms displaying various antimicrobial, cytolytic, and potassium channel-blocking activities. The relative contribution of each domain to their different activities remains to be elucidated. Here, we report the recombinant production, solution structure, and antiparasitic activity of Hge36, first identified as a naturally occurring truncated form of a Scorpine-like peptide from the venom of Hoffmannihadrurus gertschi. We also show that removing the first four residues from Hge36 renders a molecule with enhanced potassium channel-blocking and antiparasitic activities. Our results are important to rationalize the structure-function relationships of a pharmacologically versatile molecular scaffold.
Organizational Affiliation:
Departamento de Química de Biomacromoléculas, Instituto de Química, Universidad Nacional Autónoma de México, CU, Ciudad de México, México.