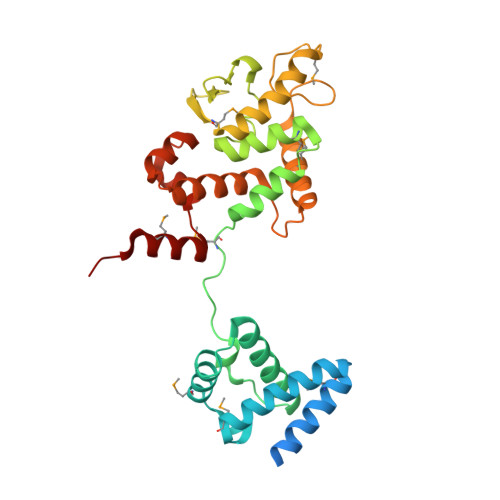
Crystal structure of Thermoplasma acidophilum XerA recombinase shows large C-shape clamp conformation and cis-cleavage mode for nucleophilic tyrosine
Jo, C.H., Kim, J., Han, A.R., Park, S.Y., Hwang, K.Y., Nam, K.H.(2016) FEBS Lett 590: 848-856
- PubMed: 26919387
- DOI: https://doi.org/10.1002/1873-3468.12109
- Primary Citation of Related Structures:
5HXY - PubMed Abstract:
Site-specific Xer recombination plays a pivotal role in reshuffling genetic information. Here, we report the 2.5 Å crystal structure of XerA from the archaean Thermoplasma acidophilum. Crystallographic data reveal a uniquely open conformational state, resulting in a C-shaped clamp with an angle of ~ 48° and a distance of 57 Å between the core-binding and the catalytic domains. The catalytic nucleophile, Tyr264, is positioned in cis-cleavage mode by XerA's C-term tail that interacts with the CAT domain of a neighboring monomer without DNA substrate. Structural comparisons of tyrosine recombinases elucidate the dynamics of Xer recombinase.
Organizational Affiliation:
Division of Biotechnology, College of Life Sciences and Biotechnology, Korea University, Seoul, Korea.