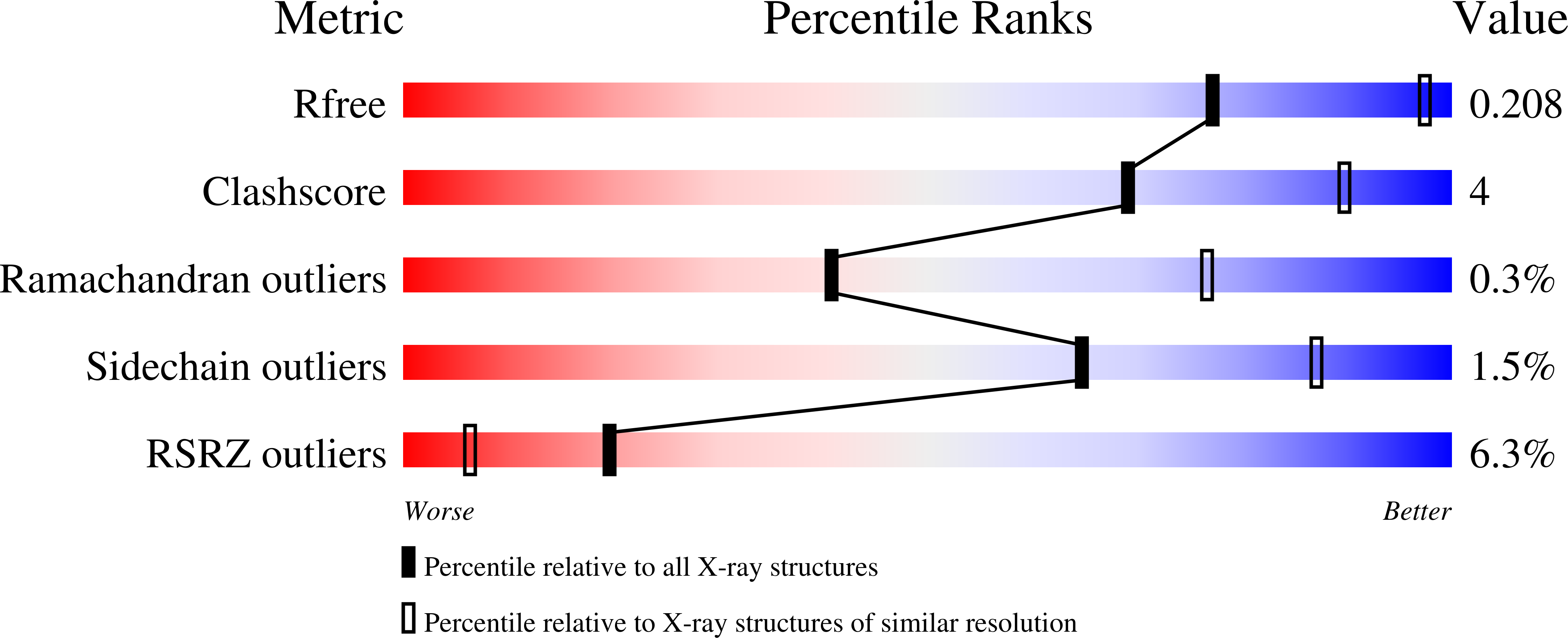
Elucidation of the molecular mechanisms of two nanobodies that inhibit thrombin-activatable fibrinolysis inhibitor activation and activated thrombin-activatable fibrinolysis inhibitor activity.
Zhou, X., Weeks, S.D., Ameloot, P., Callewaert, N., Strelkov, S.V., Declerck, P.J.(2016) J Thromb Haemost 14: 1629-1638
- PubMed: 27279497
- DOI: https://doi.org/10.1111/jth.13381
- Primary Citation of Related Structures:
5HVF, 5HVG, 5HVH - PubMed Abstract:
Essentials Thrombin-activatable fibrinolysis inhibitor (TAFI) is a risk factor for cardiovascular disorders. TAFI inhibitory nanobodies represent a promising step in developing profibrinolytic therapeutics. We have solved three crystal structures of TAFI in complex with inhibitory nanobodies. Nanobodies inhibit TAFI through distinct mechanisms and represent novel profibrinolytic leads.
Organizational Affiliation:
Department of Pharmaceutical and Pharmacologic Sciences, Laboratory for Therapeutic and Diagnostic Antibodies, KU Leuven, Belgium.