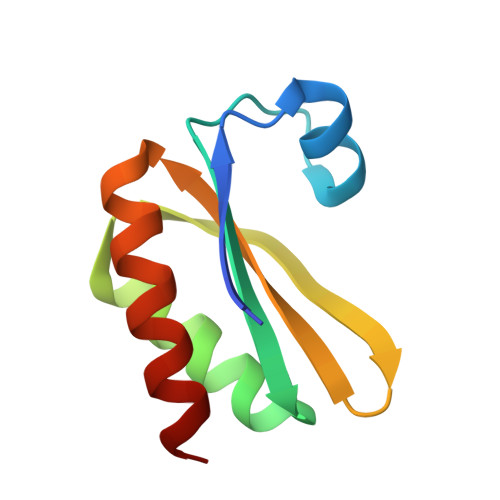
Structure of a novel 13 nm dodecahedral nanocage assembled from a redesigned bacterial microcompartment shell protein.
Jorda, J., Leibly, D.J., Thompson, M.C., Yeates, T.O.(2016) Chem Commun (Camb) 52: 5041-5044
- PubMed: 26988700
- DOI: https://doi.org/10.1039/c6cc00851h
- Primary Citation of Related Structures:
5HPN - PubMed Abstract:
We report the crystal structure of a novel 60-subunit dodecahedral cage that results from self-assembly of a re-engineered version of a natural protein (PduA) from the Pdu microcompartment shell. Biophysical data illustrate the dependence of assembly on solution conditions, opening up new applications in microcompartment studies and nanotechnology.
Organizational Affiliation:
UCLA-DOE Institute for Genomics and Proteomics, University of California, Los Angeles, CA 90095, USA. yeates@mbi.ucla.edu.