Salt-bridge modulates differential calcium-mediated ligand binding to integrin alpha 1- and alpha 2-I domains.
Brown, K.L., Banerjee, S., Feigley, A., Abe, H., Blackwell, T.S., Pozzi, A., Hudson, B.G., Zent, R.(2018) Sci Rep 8: 2916-2916
- PubMed: 29440721
- DOI: https://doi.org/10.1038/s41598-018-21231-1
- Primary Citation of Related Structures:
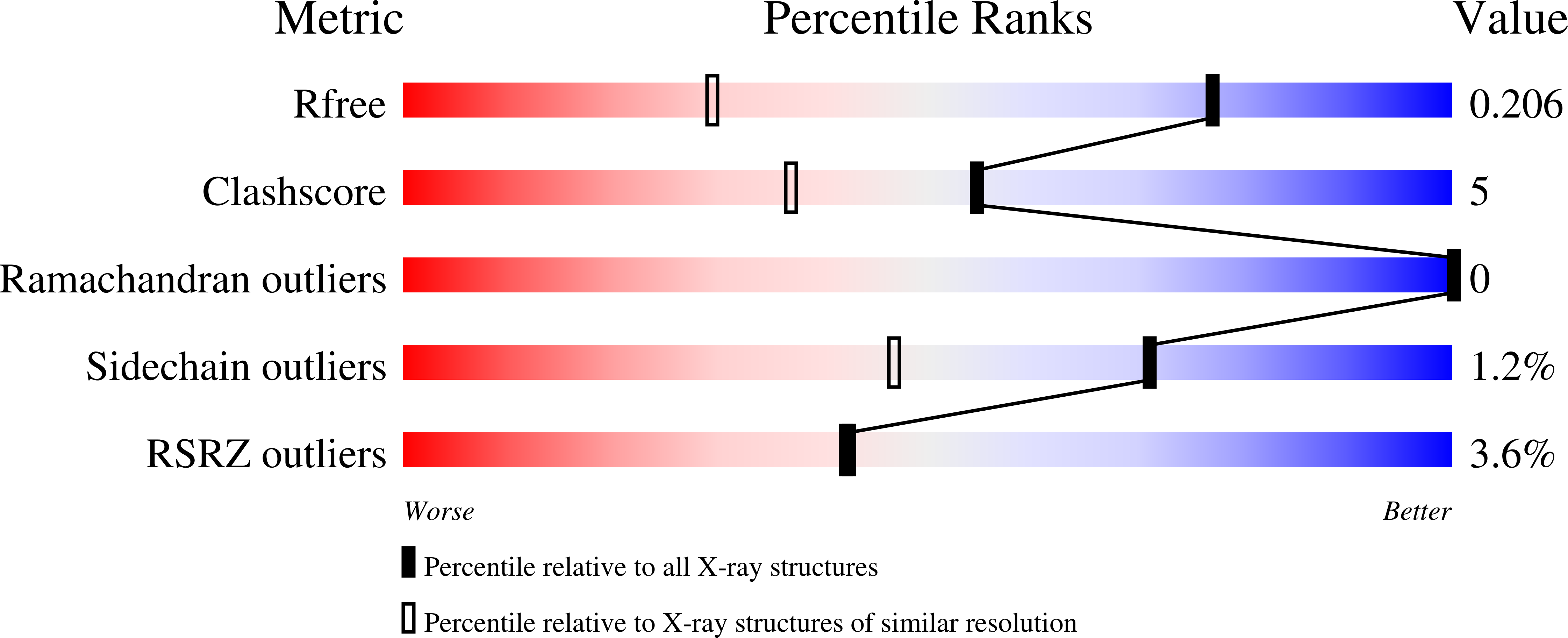
5HGJ, 5HJ2 - PubMed Abstract:
Integrins are transmembrane cell-extracellular matrix adhesion receptors that impact many cellular functions. A subgroup of integrins contain an inserted (I) domain within the α-subunits (αI) that mediate ligand recognition where function is contingent on binding a divalent cation at the metal ion dependent adhesion site (MIDAS). Ca 2+ is reported to promote α1I but inhibit α2I ligand binding. We co-crystallized individual I-domains with MIDAS-bound Ca 2+ and report structures at 1.4 and 2.15 Å resolution, respectively. Both structures are in the "closed" ligand binding conformation where Ca 2+ induces minimal global structural changes. Comparisons with Mg 2+ -bound structures reveal Mg 2+ and Ca 2+ bind α1I in a manner sufficient to promote ligand binding. In contrast, Ca 2+ is displaced in the α2I domain MIDAS by 1.4 Å relative to Mg 2+ and unable to directly coordinate all MIDAS residues. We identified an E152-R192 salt bridge hypothesized to limit the flexibility of the α2I MIDAS, thus, reducing Ca 2+ binding. A α2I E152A construct resulted in a 10,000-fold increase in Mg 2+ and Ca 2+ binding affinity while increasing binding to collagen ligands 20%. These data indicate the E152-R192 salt bridge is a key distinction in the molecular mechanism of differential ion binding of these two I domains.
Organizational Affiliation:
Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, 37232-2372, USA. kyle.l.brown@vanderbilt.edu.