Plant receptor complex at 2.35 Angstroms resolution
Ma, R., Han, Z., Hu, Z., Lin, G., Gong, X., Zhang, H., June, N., Chai, J.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| S-receptor kinase SRK9 | 405 | Brassica rapa | Mutation(s): 0 | 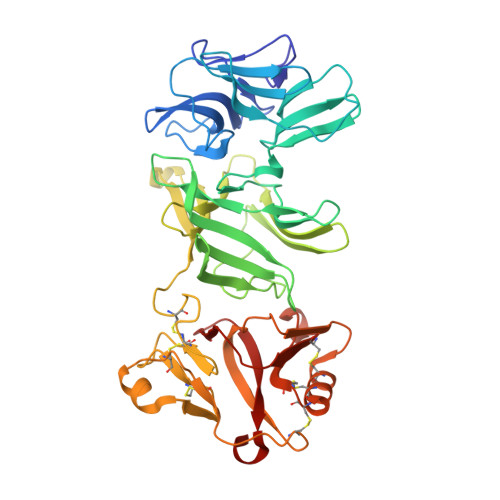 | |
UniProt | |||||
Find proteins for Q7DN95 (Brassica campestris) Explore Q7DN95 Go to UniProtKB: Q7DN95 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q7DN95 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| S-locus protein 11 | C [auth G], D [auth H] | 59 | Brassica rapa | Mutation(s): 0 Gene Names: SP11 |  |
UniProt | |||||
Find proteins for Q9ST12 (Brassica campestris) Explore Q9ST12 Go to UniProtKB: Q9ST12 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9ST12 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | E [auth G], F [auth H] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 62.249 | α = 90 |
| b = 135.121 | β = 90 |
| c = 153.907 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Science Foundation of China | China | 31130063 |
| Chinese Ministry of Science and Technology | China | 2015CB910200 |