Structure of Chorismate Mutase-like Domain of DAHPS from Bacillus subtilis Complexed with Novel Inhibitor Reveals Conformational Plasticity of Active Site.
Pratap, S., Dev, A., Kumar, V., Yadav, R., Narwal, M., Tomar, S., Kumar, P.(2017) Sci Rep 7: 6364-6364
- PubMed: 28743924
- DOI: https://doi.org/10.1038/s41598-017-06578-1
- Primary Citation of Related Structures:
5GMU, 5GO2 - PubMed Abstract:
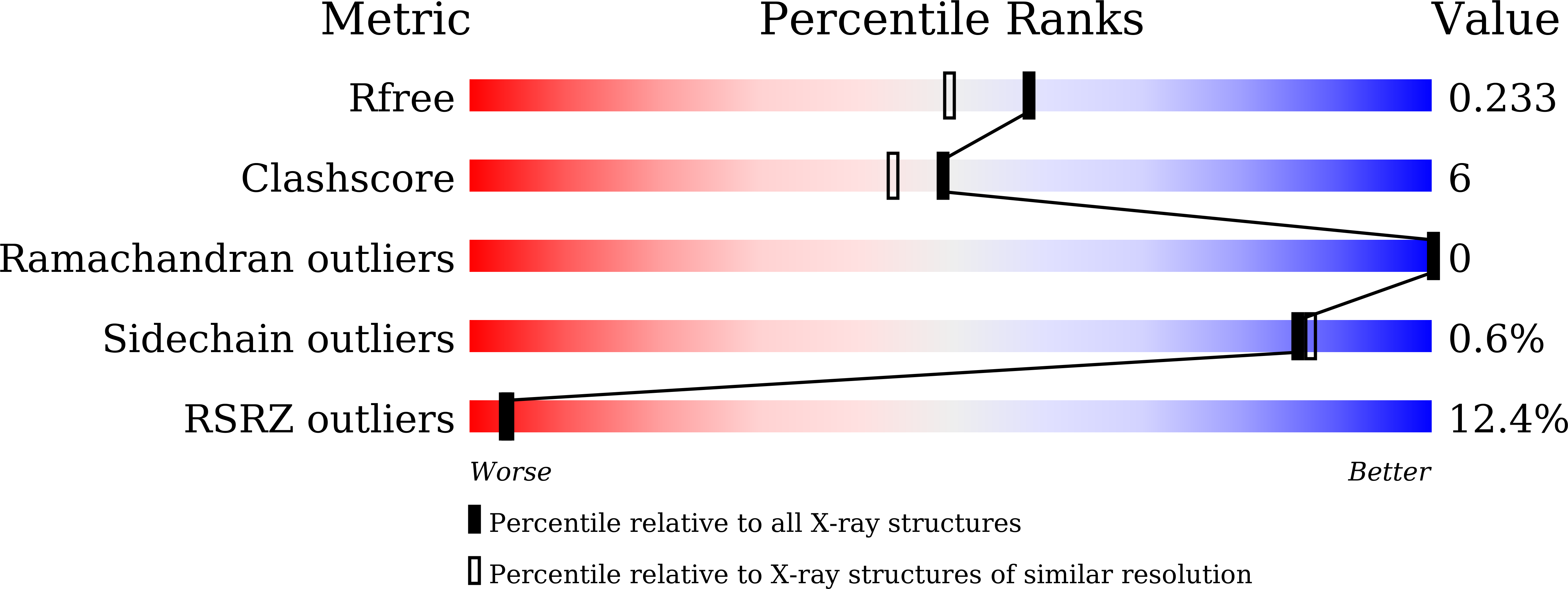
3-deoxy-D-arabino-heptulosonate-7-phosphate-synthase (DAHPS) is the first enzyme of the shikimate pathway and is responsible for the synthesis of aromatic amino acids in microorganisms. This pathway is an attractive target for antimicrobial drugs. In Bacillus subtilis, the N-terminal domain of the bifunctional DAHPS enzyme belongs to an AroQ class of chorismate mutase and is functionally homologous to the downstream AroH class chorismate mutase. This is the first structure of chorismate mutase, AroQ (BsCM_2) enzyme from Bacillus subtilis in complex with citrate and chlorogenic acid at 1.9 Å and 1.8 Å resolution, respectively. This work provides the structural basis of ligand binding into the active site of AroQ class of chorismate mutase, while accompanied by the conformational flexibility of active site loop. Molecular dynamics results showed that helix H2' undergoes uncoiling at the first turn and increases the mobility of loop L1'. The side chains of Arg45, Phe46, Arg52 and Lys76 undergo conformational changes, which may play an important role in DAHPS regulation by the formation of the domain-domain interface. Additionally, binding studies showed that the chlorogenic acid binds to BsCM_2 with a higher affinity than chorismate. These biochemical and structural findings could lead to the development of novel antimicrobial drugs.
Organizational Affiliation:
Department of Biotechnology, Indian Institute of Technology Roorkee, Roorkee, 247667, India.