Pheromone Recognition and Selectivity by ComR Proteins among Streptococcus Species.
Shanker, E., Morrison, D.A., Talagas, A., Nessler, S., Federle, M.J., Prehna, G.(2016) PLoS Pathog 12: e1005979-e1005979
- PubMed: 27907154
- DOI: https://doi.org/10.1371/journal.ppat.1005979
- Primary Citation of Related Structures:
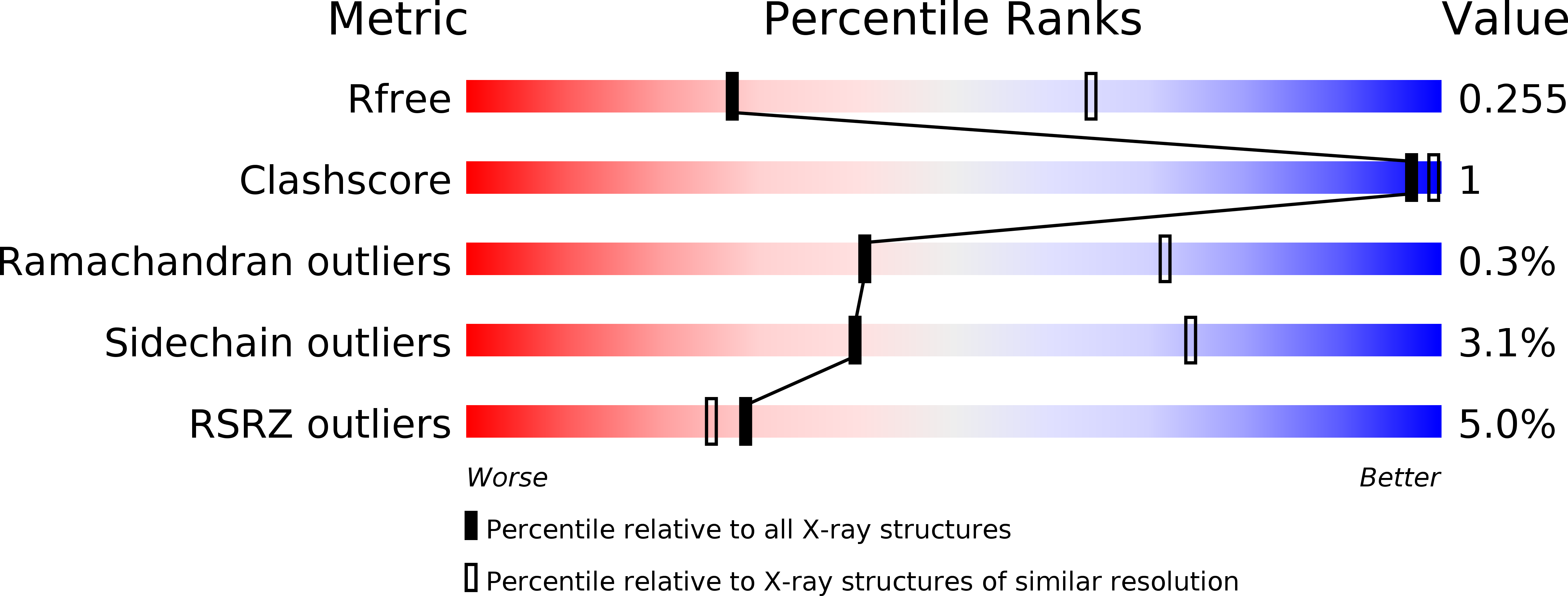
5FD4 - PubMed Abstract:
Natural transformation, or competence, is an ability inherent to bacteria for the uptake of extracellular DNA. This process is central to bacterial evolution and allows for the rapid acquirement of new traits, such as antibiotic resistance in pathogenic microorganisms. For the Gram-positive bacteria genus Streptococcus, genes required for competence are under the regulation of quorum sensing (QS) mediated by peptide pheromones. One such system, ComRS, consists of a peptide (ComS) that is processed (XIP), secreted, and later imported into the cytoplasm, where it binds and activates the transcription factor ComR. ComR then engages in a positive feedback loop for the expression of ComS and the alternative sigma-factor SigX. Although ComRS are present in the majority of Streptococcus species, the sequence of both ComS/XIP and ComR diverge significantly, suggesting a mechanism for species-specific communication. To study possible cross-talk between streptococcal species in the regulation of competence, and to explore in detail the molecular interaction between ComR and XIP we undertook an interdisciplinary approach. We developed a 'test-bed' assay to measure the activity of different ComR proteins in response to cognate and heterologous XIP peptides in vivo, revealing distinct ComR classes of strict, intermediate, and promiscuous specificity among species. We then solved an X-ray crystal structure of ComR from S. suis to further understand the interaction with XIP and to search for structural features in ComR proteins that may explain XIP recognition. Using the structure as a guide, we probed the apo conformation of the XIP-binding pocket by site-directed mutagenesis, both in test-bed cultures and biochemically in vitro. In alignments with ComR proteins from other species, we find that the pocket is lined by a variable and a conserved face, where residues of the conserved face contribute to ligand binding and the variable face discriminate among XIP peptides. Together, our results not only provide a model for XIP recognition and specificity, but also allow for the prediction of novel XIP peptides that induce ComR activity.
Organizational Affiliation:
Department of Medicinal Chemistry and Pharmacognosy, University of Illinois at Chicago, Chicago, IL, United States of America.