Crystal structure of SF216 from Shigella flexneri strain 5a
Lee, Y.-S., Seok, S.-H., Kim, H.-N., An, J.-G., Chung, K.Y., Won, H.-S., Seo, M.-D.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Uncharacterized protein | 136 | Shigella flexneri 5a str. M90T | Mutation(s): 0 Gene Names: SF5M90T_216 | 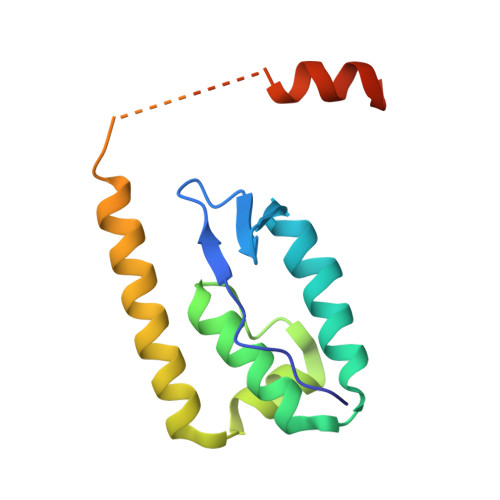 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | E [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| MG Query on MG | D [auth A] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 87.399 | α = 90 |
| b = 50.411 | β = 104.1 |
| c = 99.921 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| PHENIX | model building |
| PHENIX | phasing |
| HKL-2000 | data scaling |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Research Foundation of Korea | Korea, Republic Of | 2012R1A1A1039738 |
| National Research Foundation of Korea | Korea, Republic Of | 2014R1A1A2054691 |