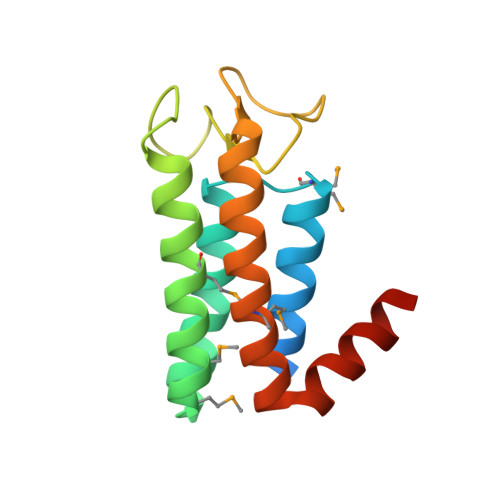
Structural studies ofAcidianustailed spindle virus reveal a structural paradigm used in the assembly of spindle-shaped viruses.
Hochstein, R., Bollschweiler, D., Dharmavaram, S., Lintner, N.G., Plitzko, J.M., Bruinsma, R., Engelhardt, H., Young, M.J., Klug, W.S., Lawrence, C.M.(2018) Proc Natl Acad Sci U S A 115: 2120-2125
- PubMed: 29440399
- DOI: https://doi.org/10.1073/pnas.1719180115
- Primary Citation of Related Structures:
5EQW - PubMed Abstract:
The spindle-shaped virion morphology is common among archaeal viruses, where it is a defining characteristic of many viral families. However, structural heterogeneity intrinsic to spindle-shaped viruses has seriously hindered efforts to elucidate the molecular architecture of these lemon-shaped capsids. We have utilized a combination of cryo-electron microscopy and X-ray crystallography to study Acidianus tailed spindle virus (ATSV). These studies reveal the architectural principles that underlie assembly of a spindle-shaped virus. Cryo-electron tomography shows a smooth transition from the spindle-shaped capsid into the tubular-shaped tail and allows low-resolution structural modeling of individual virions. Remarkably, higher-dose 2D micrographs reveal a helical surface lattice in the spindle-shaped capsid. Consistent with this, crystallographic studies of the major capsid protein reveal a decorated four-helix bundle that packs within the crystal to form a four-start helical assembly with structural similarity to the tube-shaped tail structure of ATSV and other tailed, spindle-shaped viruses. Combined, this suggests that the spindle-shaped morphology of the ATSV capsid is formed by a multistart helical assembly with a smoothly varying radius and allows construction of a pseudoatomic model for the lemon-shaped capsid that extends into a tubular tail. The potential advantages that this novel architecture conveys to the life cycle of spindle-shaped viruses, including a role in DNA ejection, are discussed.
Organizational Affiliation:
Department of Microbiology and Immunology, Montana State University, Bozeman, MT 59717.