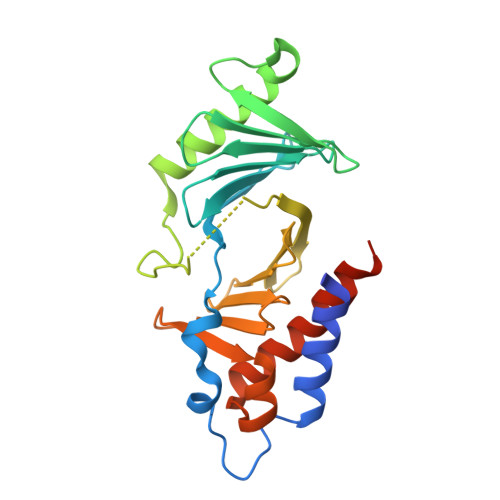
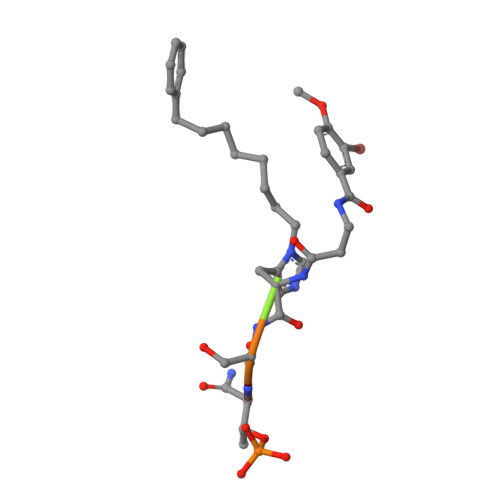
Structural basis for recognition of Emi2 by Polo-like kinase 1 and development of peptidomimetics blocking oocyte maturation and fertilization.
Jia, J.L., Han, Y.H., Kim, H.C., Ahn, M., Kwon, J.W., Luo, Y., Gunasekaran, P., Lee, S.J., Lee, K.S., Bang, J.K., Kim, N.H., Namgoong, S.(2015) Sci Rep 5: 14626-14626
- PubMed: 26459104
- DOI: https://doi.org/10.1038/srep14626
- Primary Citation of Related Structures:
5DMS, 5DMV, 5DNJ - PubMed Abstract:
In a mammalian oocyte, completion of meiosis is suspended until fertilization by a sperm, and the cell cycle is arrested by a biochemical activity called cytostatic factor (CSF). Emi2 is one of the CSFs, and it maintains the protein level of maturation promoting factor (MPF) by inhibiting ubiquitin ligase anaphase promoting complex/cyclosome (APC/C). Degradation of Emi2 via ubiquitin-mediated proteolysis after fertilization requires phosphorylation by Polo-like kinase 1 (Plk1). Therefore, recognition and phosphorylation of Emi2 by Plk1 are crucial steps for cell cycle resumption, but the binding mode of Emi2 and Plk1 is poorly understood. Using biochemical assays and X-ray crystallography, we found that two phosphorylated threonines (Thr(152) and Thr(176)) in Emi2 are each responsible for the recruitment of one Plk1 molecule by binding to its C-terminal polo box domain (PBD). We also found that meiotic maturation and meiosis resumption via parthenogenetic activation were impaired when Emi2 interaction with Plk1-PBD was blocked by a peptidomimetic called 103-8. Because of the inherent promiscuity of kinase inhibitors, our results suggest that targeting PBD of Plk1 may be an effective strategy for the development of novel and specific contraceptive agents that block oocyte maturation and/or fertilization.
Organizational Affiliation:
Department of Animal Sciences, Chungbuk National University, Republic of Korea.