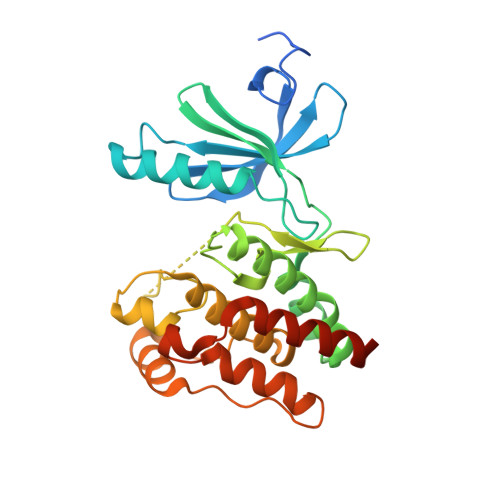
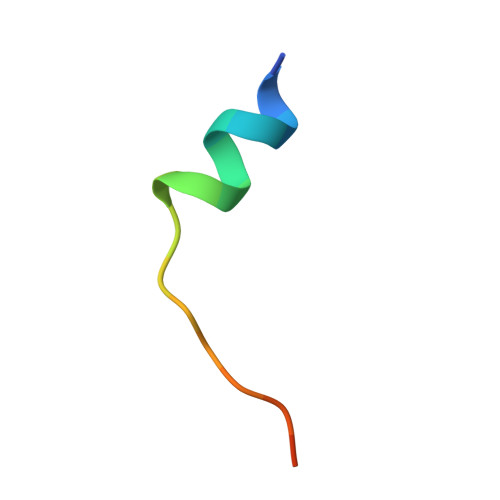
Mechanistic basis of Nek7 activation through Nek9 binding and induced dimerization.
Haq, T., Richards, M.W., Burgess, S.G., Gallego, P., Yeoh, S., O'Regan, L., Reverter, D., Roig, J., Fry, A.M., Bayliss, R.(2015) Nat Commun 6: 8771-8771
- PubMed: 26522158
- DOI: https://doi.org/10.1038/ncomms9771
- Primary Citation of Related Structures:
5DE2 - PubMed Abstract:
Mitotic spindle assembly requires the regulated activities of protein kinases such as Nek7 and Nek9. Nek7 is autoinhibited by the protrusion of Tyr97 into the active site and activated by the Nek9 non-catalytic C-terminal domain (CTD). CTD binding apparently releases autoinhibition because mutation of Tyr97 to phenylalanine increases Nek7 activity independently of Nek9. Here we find that self-association of the Nek9-CTD is needed for Nek7 activation. We map the minimal Nek7 binding region of Nek9 to residues 810-828. A crystal structure of Nek7(Y97F) bound to Nek9(810-828) reveals a binding site on the C-lobe of the Nek7 kinase domain. Nek7(Y97F) crystallizes as a back-to-back dimer between kinase domain N-lobes, in which the specific contacts within the interface are coupled to the conformation of residue 97. Hence, we propose that the Nek9-CTD activates Nek7 through promoting back-to-back dimerization that releases the autoinhibitory tyrosine residue, a mechanism conserved in unrelated kinase families.
Organizational Affiliation:
Department of Biochemistry, University of Leicester, Lancaster Road, Leicester LE1 9HN, UK.