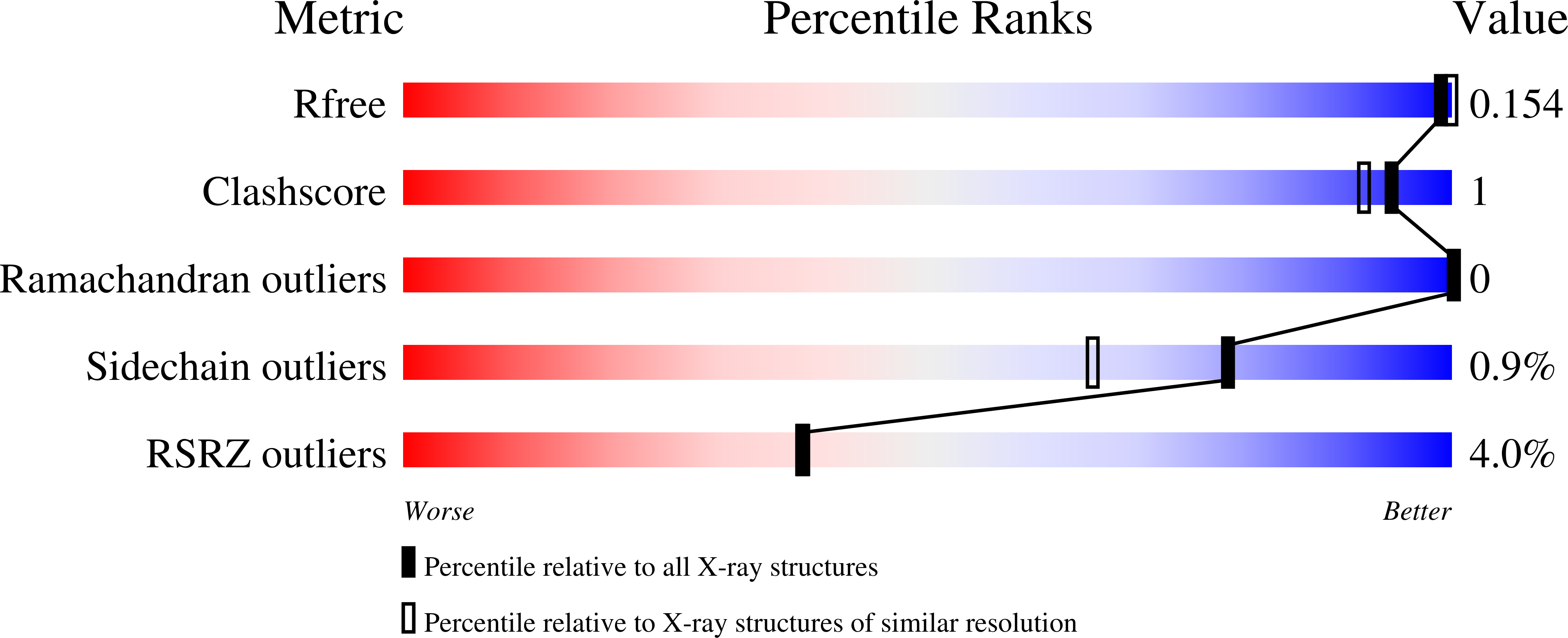
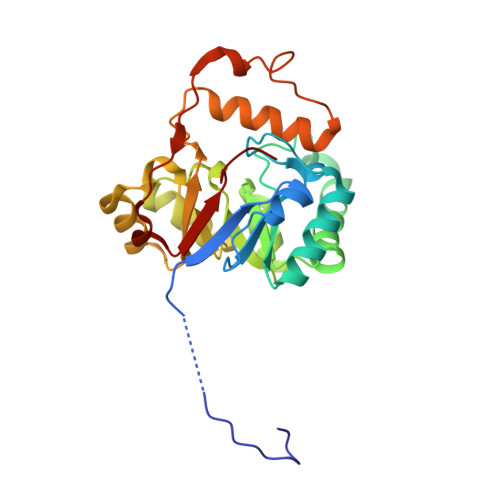
The first crystal structure of the peptidase domain of the U32 peptidase family.
Schacherl, M., Montada, A.A., Brunstein, E., Baumann, U.(2015) Acta Crystallogr D Biol Crystallogr 71: 2505-2512
- PubMed: 26627657
- DOI: https://doi.org/10.1107/S1399004715019549
- Primary Citation of Related Structures:
5D88 - PubMed Abstract:
The U32 family is a collection of over 2500 annotated peptidases in the MEROPS database with unknown catalytic mechanism. They mainly occur in bacteria and archaea, but a few representatives have also been identified in eukarya. Many of the U32 members have been linked to pathogenicity, such as proteins from Helicobacter and Salmonella. The first crystal structure analysis of a U32 catalytic domain from Methanopyrus kandleri (gene mk0906) reveals a modified (βα)8 TIM-barrel fold with some unique features. The connecting segment between strands β7 and β8 is extended and helix α7 is located on top of the C-terminal end of the barrel body. The protein exhibits a dimeric quaternary structure in which a zinc ion is symmetrically bound by histidine and cysteine side chains from both monomers. These residues reside in conserved sequence motifs. No typical proteolytic motifs are discernible in the three-dimensional structure, and biochemical assays failed to demonstrate proteolytic activity. A tunnel in which an acetate ion is bound is located in the C-terminal part of the β-barrel. Two hydrophobic grooves lead to a tunnel at the C-terminal end of the barrel in which an acetate ion is bound. One of the grooves binds to a Strep-Tag II of another dimer in the crystal lattice. Thus, these grooves may be binding sites for hydrophobic peptides or other ligands.
Organizational Affiliation:
Department of Chemistry, Institute of Biochemistry, University of Cologne, Otto-Fischer-Strasse 12-14, D-50674 Cologne, Germany.