Proline dipeptidase from Deinococcus radiodurans R1 at 1.45 Angstrom resolution
Kumar, A., Are, V., Ghosh, B., Jamdar, S., Makde, R.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Proline dipeptidase | 349 | Deinococcus radiodurans R1 = ATCC 13939 = DSM 20539 | Mutation(s): 0 Gene Names: DR_1246 | 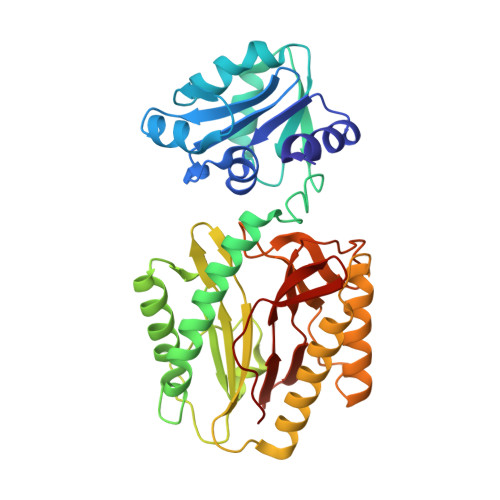 | |
UniProt | |||||
Find proteins for Q9RUY4 (Deinococcus radiodurans (strain ATCC 13939 / DSM 20539 / JCM 16871 / CCUG 27074 / LMG 4051 / NBRC 15346 / NCIMB 9279 / VKM B-1422 / R1)) Explore Q9RUY4 Go to UniProtKB: Q9RUY4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9RUY4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PO4 Query on PO4 | D [auth A], E [auth A], F [auth A], G [auth A], H [auth A] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| MN Query on MN | B [auth A] | MANGANESE (II) ION Mn WAEMQWOKJMHJLA-UHFFFAOYSA-N |  | ||
| NA Query on NA | C [auth A] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 60.619 | α = 90 |
| b = 60.619 | β = 90 |
| c = 202.784 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| Coot | model building |
| PHENIX | model building |
| PHASER | phasing |
| Aimless | data scaling |
| XDS | data processing |
| MAR345dtb | data collection |