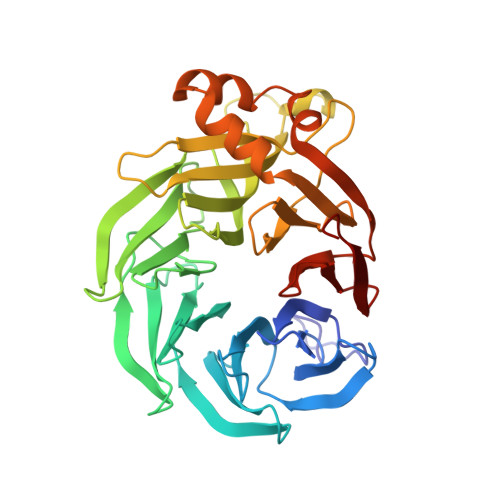
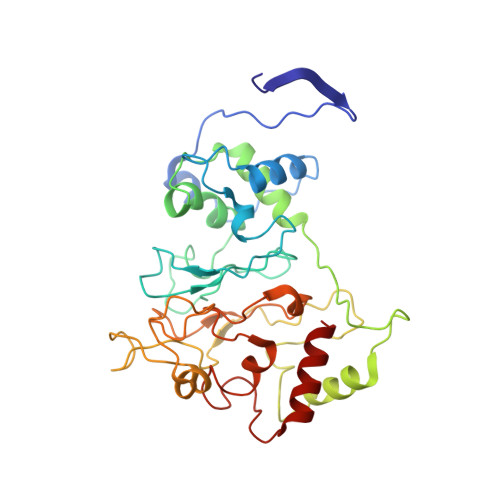
The inner workings of the hydrazine synthase multiprotein complex.
Dietl, A., Ferousi, C., Maalcke, W.J., Menzel, A., de Vries, S., Keltjens, J.T., Jetten, M.S., Kartal, B., Barends, T.R.(2015) Nature 527: 394-397
- PubMed: 26479033
- DOI: https://doi.org/10.1038/nature15517
- Primary Citation of Related Structures:
5C2V, 5C2W - PubMed Abstract:
Anaerobic ammonium oxidation (anammox) has a major role in the Earth's nitrogen cycle and is used in energy-efficient wastewater treatment. This bacterial process combines nitrite and ammonium to form dinitrogen (N2) gas, and has been estimated to synthesize up to 50% of the dinitrogen gas emitted into our atmosphere from the oceans. Strikingly, the anammox process relies on the highly unusual, extremely reactive intermediate hydrazine, a compound also used as a rocket fuel because of its high reducing power. So far, the enzymatic mechanism by which hydrazine is synthesized is unknown. Here we report the 2.7 Å resolution crystal structure, as well as biophysical and spectroscopic studies, of a hydrazine synthase multiprotein complex isolated from the anammox organism Kuenenia stuttgartiensis. The structure shows an elongated dimer of heterotrimers, each of which has two unique c-type haem-containing active sites, as well as an interaction point for a redox partner. Furthermore, a system of tunnels connects these active sites. The crystal structure implies a two-step mechanism for hydrazine synthesis: a three-electron reduction of nitric oxide to hydroxylamine at the active site of the γ-subunit and its subsequent condensation with ammonia, yielding hydrazine in the active centre of the α-subunit. Our results provide the first, to our knowledge, detailed structural insight into the mechanism of biological hydrazine synthesis, which is of major significance for our understanding of the conversion of nitrogenous compounds in nature.
Organizational Affiliation:
Department of Biomolecular Mechanisms, Max Planck Institute for Medical Research, 69120 Heidelberg, Germany.