Structural basis of G-protein target alternation of MgcRacGAP by phospholylation
Murayama, K., Kato-Murayama, M., Hosaka, T., Kawashima, T., Kitamura, T., Yokoyama, S., Shirouzu, M.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Rac GTPase-activating protein 1 | 208 | Homo sapiens | Mutation(s): 0 Gene Names: RACGAP1, KIAA1478, MGCRACGAP | 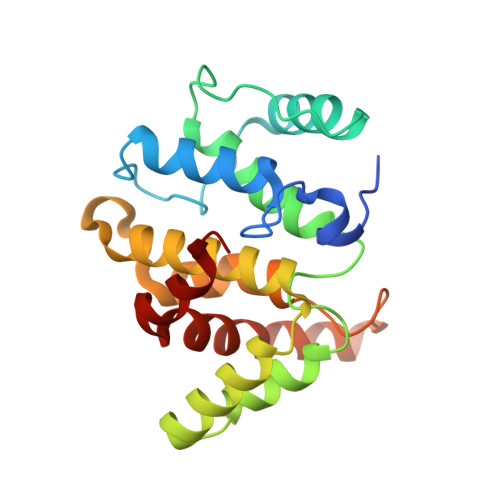 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9H0H5 (Homo sapiens) Explore Q9H0H5 Go to UniProtKB: Q9H0H5 | |||||
PHAROS: Q9H0H5 GTEx: ENSG00000161800 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9H0H5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cell division control protein 42 homolog | 198 | Mus musculus | Mutation(s): 0 Gene Names: Cdc42 | 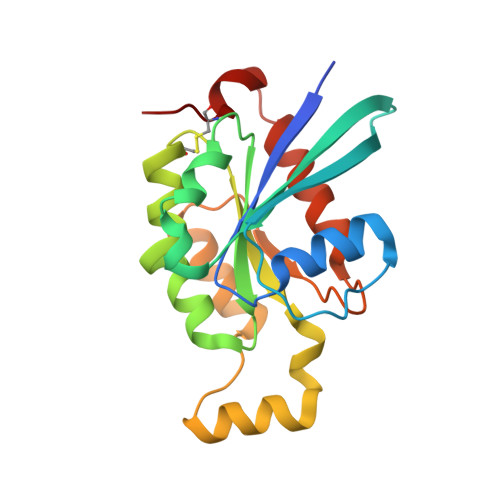 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P60766 (Mus musculus) Explore P60766 Go to UniProtKB: P60766 | |||||
IMPC: MGI:106211 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P60766 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GDP Query on GDP | D [auth B] | GUANOSINE-5'-DIPHOSPHATE C10 H15 N5 O11 P2 QGWNDRXFNXRZMB-UUOKFMHZSA-N |  | ||
| AF3 Query on AF3 | E [auth B] | ALUMINUM FLUORIDE Al F3 KLZUFWVZNOTSEM-UHFFFAOYSA-K |  | ||
| MG Query on MG | C [auth B] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 43.324 | α = 90 |
| b = 74.237 | β = 96.67 |
| c = 55.423 | γ = 90 |
| Software Name | Purpose |
|---|---|
| CNS | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| MOLREP | phasing |