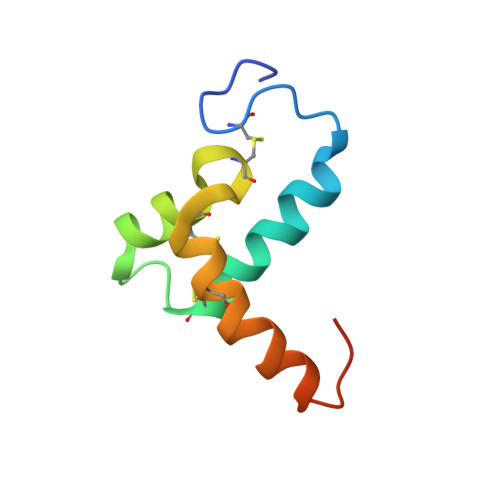
Crystal structure of a crustacean hyperglycemic hormone (CHH) precursor suggests structural variety in the C-terminal regions of CHH superfamily members.
Tsutsui, N., Sakamoto, T., Arisaka, F., Tanokura, M., Nagasawa, H., Nagata, K.(2016) FEBS J 283: 4325-4339
- PubMed: 27743429
- DOI: https://doi.org/10.1111/febs.13926
- Primary Citation of Related Structures:
5B5I - PubMed Abstract:
The crustacean hyperglycemic hormone (CHH) is one of the major hormones in crustaceans, and peptides belonging to the CHH superfamily have been found in diverse ecdysozoans. Although the basic function of CHH is to control energy metabolism, it also plays various roles in crustacean species, such as in molting and vitellogenesis. Here, we present the crystal structure of Pej-SGP-I-Gly, a partially active precursor of CHH from the kuruma prawn Marsupenaeus japonicus, which has an additional Gly residue in place of the C-terminal amide group of the mature Pej-SGP-I. The 1.6-angstrom crystal structure showed not only the common CHH superfamily scaffold comprising three α-helices, three disulfide bridges, and a hydrophobic core but also revealed that the C-terminal part has a variant backbone fold that is specific to Pej-SGP-I-Gly. The α-helix 4 of Pej-SGP-I-Gly was much longer than that of molt-inhibiting hormone (Pej-MIH) from the same species, and as a result, the following C-terminal helix, corresponding to α-helix 5 in MIH, was not formed. Unlike monomeric Pej-MIH, Pej-SGP-I-Gly forms a homodimer in the crystal structure via its unique α-helix 4. The unexpected dissimilar folds between Pej-SGP-I-Gly and Pej-MIH appear to be the result of their distinct C-terminal amino acid sequences. Variations in amino acid sequences and lengths and the resulting variety of backbone folds allow the C-terminal and sterically adjoining regions to confer different hormonal activities in diverse CHH superfamily members.
Organizational Affiliation:
Ushimado Marine Institute, Faculty of Science, Okayama University, Setouchi, Japan.