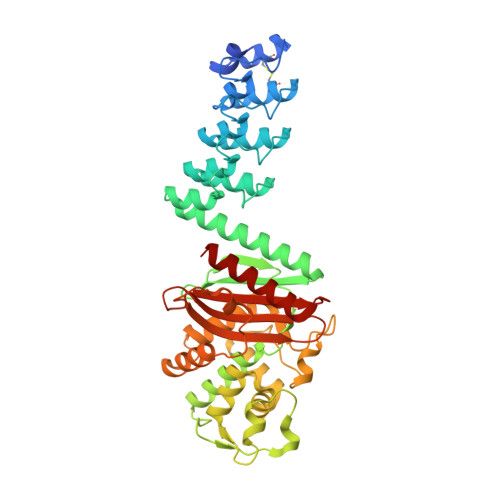
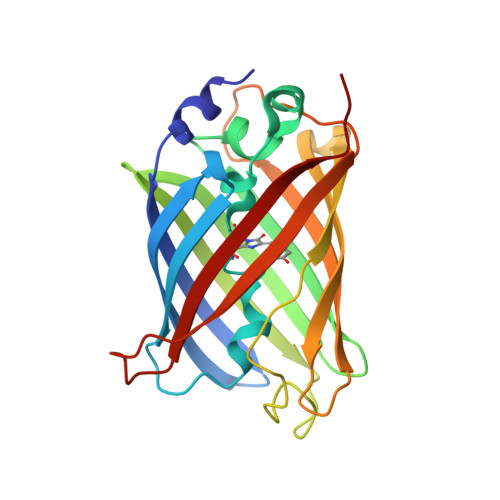
Darpin-Based Crystallization Chaperones Exploit Molecular Geometry as a Screening Dimension in Protein Crystallography
Batyuk, A., Wu, Y., Honegger, A., Heberling, M., Plueckthun, A.(2016) J Mol Biol 428: 1574
- PubMed: 26975886
- DOI: https://doi.org/10.1016/j.jmb.2016.03.002
- Primary Citation of Related Structures:
5AQ7, 5AQ8, 5AQ9, 5AQA, 5AQB - PubMed Abstract:
DARPin libraries, based on a Designed Ankyrin Repeat Protein consensus framework, are a rich source of binding partners for a wide variety of proteins. Their modular structure, stability, ease of in vitro selection and high production yields make DARPins an ideal starting point for further engineering. The X-ray structures of around 30 different DARPin complexes demonstrate their ability to facilitate crystallization of their target proteins by restricting flexibility and preventing undesired interactions of the target molecule. However, their small size (18 kDa), very hydrophilic surface and repetitive structure can limit the DARPins' ability to provide essential crystal contacts and their usefulness as a search model for addressing the crystallographic phase problem in molecular replacement. To optimize DARPins for their application as crystallization chaperones, rigid domain-domain fusions of the DARPins to larger proteins, proven to yield high-resolution crystal structures, were generated. These fusions were designed in such a way that they affect only one of the terminal capping repeats of the DARPin and do not interfere with residues involved in target binding, allowing to exchange at will the binding specificities of the DARPin in the fusion construct. As a proof of principle, we designed rigid fusions of a stabilized version of Escherichia coli TEM-1 β-lactamase to the C-terminal capping repeat of various DARPins in six different relative domain orientations. Five crystal structures representing four different fusion constructs, alone or in complex with the cognate target, show the predicted relative domain orientations and prove the validity of the concept.
Organizational Affiliation:
Department of Biochemistry, University of Zurich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland.