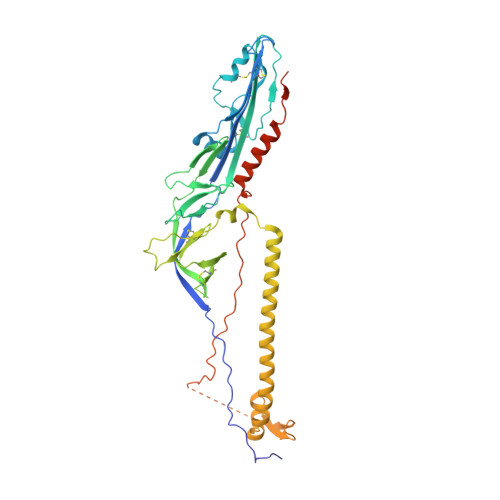
Postfusion structure of human-infecting Bourbon virus envelope glycoprotein.
Bai, C., Qi, J., Wu, Y., Wang, X., Gao, G.F., Peng, R., Gao, F.(2019) J Struct Biol 208: 99-106
- PubMed: 31419524
- DOI: https://doi.org/10.1016/j.jsb.2019.08.005
- Primary Citation of Related Structures:
5ZKX - PubMed Abstract:
Thogotoviruses are important zoonotic viruses infecting a variety of domestic animals, as well as humans. Among these viruses, Bourbon virus (BRBV) is one of the several human-infecting members, which emerged in the US in recent years and caused human deaths. Here, we report the crystal structure of the BRBV envelope glycoprotein in the postfusion conformation. The structure adopts the typical fold of a class III viral fusion protein and displays an extensive positively charged electrostatic potential pattern, which resembles the glycoprotein of Dhori virus and is consistent with our previous predictions. In addition, compared to other previously defined class III viral fusion proteins, the structures of all thogotovirus glycoproteins and homologs are more similar to herpes virus glycoprotein Bs than to the rhabdovirus G proteins. Thus, class III viral fusion proteins are quite diverse in structure, and sub-classes may have developed during evolution.
Organizational Affiliation:
CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China; Central Laboratory, Chinese Medicine Hospital of Shanxi Province, Taiyuan 030012, China.