human 199.16 TCR in complex with Melan-A/MART-1 (26-35) peptide and HLA-A2
Exertier, C., Reiser, J.-B., Housset, D.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| HLA class I histocompatibility antigen, A-2 alpha chain | A [auth H] | 276 | Homo sapiens | Mutation(s): 0 Gene Names: HLA-A, HLAA | 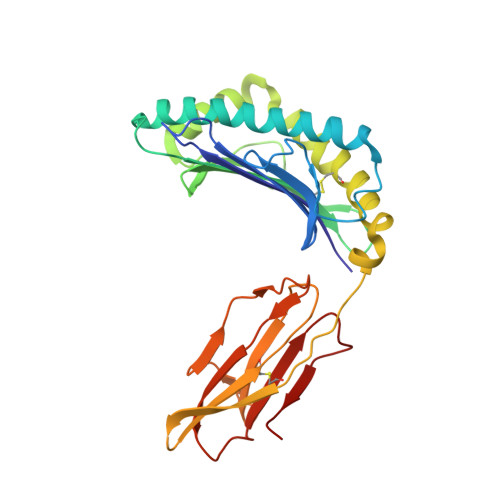 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P04439 (Homo sapiens) Explore P04439 Go to UniProtKB: P04439 | |||||
PHAROS: P04439 GTEx: ENSG00000206503 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P04439 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Beta-2-microglobulin | B [auth L] | 100 | Homo sapiens | Mutation(s): 0 Gene Names: B2M, CDABP0092, HDCMA22P | 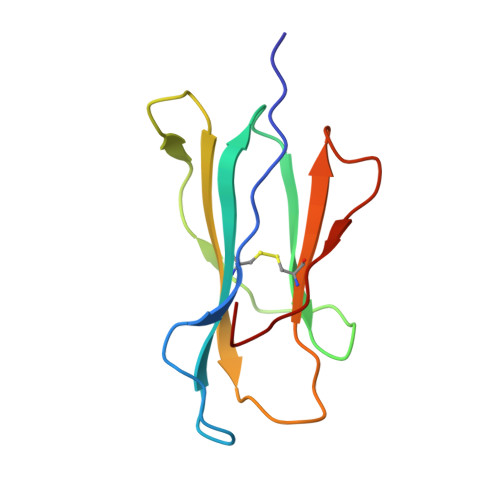 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P61769 (Homo sapiens) Explore P61769 Go to UniProtKB: P61769 | |||||
PHAROS: P61769 GTEx: ENSG00000166710 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P61769 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Melanoma antigen recognized by T-cells 1 | C [auth P] | 10 | Homo sapiens | Mutation(s): 0 | 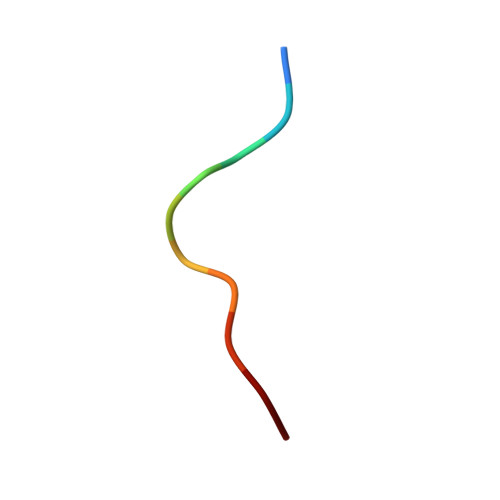 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q16655 (Homo sapiens) Explore Q16655 Go to UniProtKB: Q16655 | |||||
PHAROS: Q16655 GTEx: ENSG00000120215 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q16655 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| T-cell receptor alpha variable 12-2,T-cell receptor alpha joining 45,T-cell receptor alpha chain C region | D [auth A] | 211 | Homo sapiens | Mutation(s): 1 Gene Names: TRAV12-2, TRAJ45, TRAC | 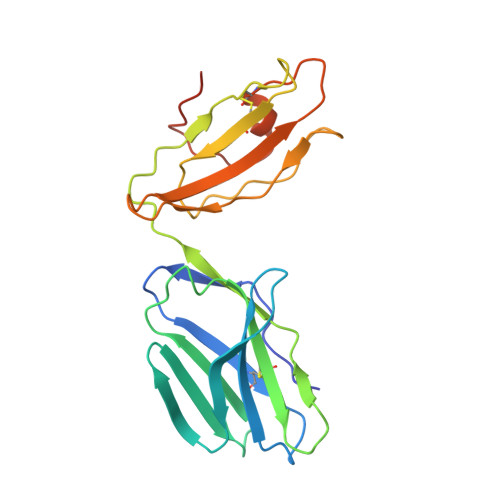 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P01848 (Homo sapiens) Explore P01848 Go to UniProtKB: P01848 | |||||
PHAROS: P01848 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P01848 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 5 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| T-cell receptor beta variable 19,TRB protein | E [auth B] | 251 | Homo sapiens | Mutation(s): 0 Gene Names: TCRBV17S1A1T, TRBV19, TRB |  |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for A0A075B6N1 (Homo sapiens) Explore A0A075B6N1 Go to UniProtKB: A0A075B6N1 | |||||
PHAROS: A0A075B6N1 GTEx: ENSG00000211746 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A075B6N1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NA Query on NA | F [auth B] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 225.18 | α = 90 |
| b = 49.72 | β = 97.69 |
| c = 95.72 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| XSCALE | data scaling |
| PHASER | phasing |