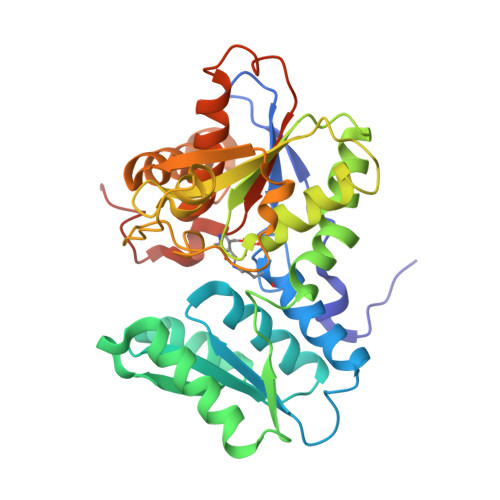
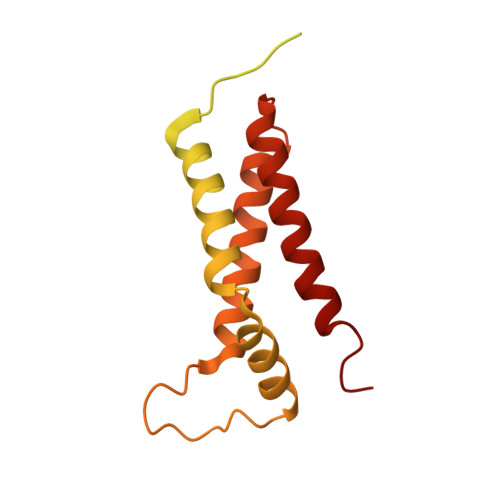
Unraveling the essential role of CysK in CDI toxin activation.
Johnson, P.M., Beck, C.M., Morse, R.P., Garza-Sanchez, F., Low, D.A., Hayes, C.S., Goulding, C.W.(2016) Proc Natl Acad Sci U S A 113: 9792-9797
- PubMed: 27531961
- DOI: https://doi.org/10.1073/pnas.1607112113
- Primary Citation of Related Structures:
5J43, 5J5V - PubMed Abstract:
Contact-dependent growth inhibition (CDI) is a widespread mechanism of bacterial competition. CDI(+) bacteria deliver the toxic C-terminal region of contact-dependent inhibition A proteins (CdiA-CT) into neighboring target bacteria and produce CDI immunity proteins (CdiI) to protect against self-inhibition. The CdiA-CT(EC536) deployed by uropathogenic Escherichia coli 536 (EC536) is a bacterial toxin 28 (Ntox28) domain that only exhibits ribonuclease activity when bound to the cysteine biosynthetic enzyme O-acetylserine sulfhydrylase A (CysK). Here, we present crystal structures of the CysK/CdiA-CT(EC536) binary complex and the neutralized ternary complex of CysK/CdiA-CT/CdiI(EC536) CdiA-CT(EC536) inserts its C-terminal Gly-Tyr-Gly-Ile peptide tail into the active-site cleft of CysK to anchor the interaction. Remarkably, E. coli serine O-acetyltransferase uses a similar Gly-Asp-Gly-Ile motif to form the "cysteine synthase" complex with CysK. The cysteine synthase complex is found throughout bacteria, protozoa, and plants, indicating that CdiA-CT(EC536) exploits a highly conserved protein-protein interaction to promote its toxicity. CysK significantly increases CdiA-CT(EC536) thermostability and is required for toxin interaction with tRNA substrates. These observations suggest that CysK stabilizes the toxin fold, thereby organizing the nuclease active site for substrate recognition and catalysis. By contrast, Ntox28 domains from Gram-positive bacteria lack C-terminal Gly-Tyr-Gly-Ile motifs, suggesting that they do not interact with CysK. We show that the Ntox28 domain from Ruminococcus lactaris is significantly more thermostable than CdiA-CT(EC536), and its intrinsic tRNA-binding properties support CysK-independent nuclease activity. The striking differences between related Ntox28 domains suggest that CDI toxins may be under evolutionary pressure to maintain low global stability.
Organizational Affiliation:
Department of Molecular Biology and Biochemistry, University of California, Irvine, CA 92697;