Crystal structure of N-acetyltransferase from Staphylococcus aureus.
Srivastava, P., Khandokar, Y., Forwood, J.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Diamine N-acetyltransferase | 168 | Staphylococcus aureus | Mutation(s): 0 Gene Names: res, speG, AL498_11440, ERS092844_02726, ERS195423_02759, R114_33, R92_33 EC: 2.3.1.57 | 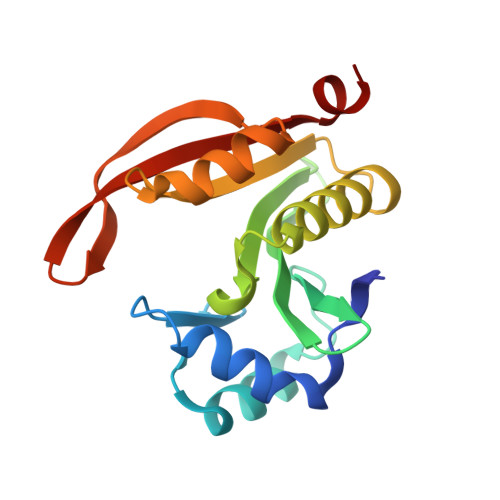 | |
UniProt | |||||
Find proteins for U5NVV0 (Staphylococcus aureus) Explore U5NVV0 Go to UniProtKB: U5NVV0 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | U5NVV0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 107.95 | α = 90 |
| b = 107.95 | β = 90 |
| c = 65.23 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| iMOSFLM | data reduction |
| Aimless | data scaling |
| PHENIX | phasing |