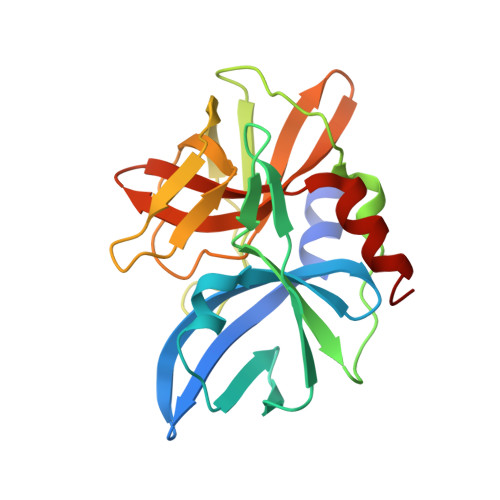
Crystal structure of the 3C protease from Southern African Territories type 2 foot-and-mouth disease virus.
Yang, J., Leen, E.N., Maree, F.F., Curry, S.(2016) PeerJ 4: e1964-e1964
- PubMed: 27168976
- DOI: https://doi.org/10.7717/peerj.1964
- Primary Citation of Related Structures:
5HM2 - PubMed Abstract:
The replication of foot-and-mouth disease virus (FMDV) is dependent on the virus-encoded 3C protease (3C(pro)). As in other picornaviruses, 3C(pro) performs most of the proteolytic processing of the polyprotein expressed from the large open reading frame in the RNA genome of the virus. Previous work revealed that the 3C(pro) from serotype A-one of the seven serotypes of FMDV-adopts a trypsin-like fold. On the basis of capsid sequence comparisons the FMDV serotypes are grouped into two phylogenetic clusters, with O, A, C, and Asia 1 in one, and the three Southern African Territories serotypes, (SAT-1, SAT-2 and SAT-3) in another, a grouping pattern that is broadly, but not rigidly, reflected in 3C(pro) amino acid sequences. We report here the cloning, expression and purification of 3C proteases from four SAT serotype viruses (SAT2/GHA/8/91, SAT1/NIG/5/81, SAT1/UGA/1/97, and SAT2/ZIM/7/83) and the crystal structure at 3.2 Å resolution of 3C(pro) from SAT2/GHA/8/91.
Organizational Affiliation:
Departmet of Life Sciences, Imperial College , London , United Kingdom.