Glycoside hydrolase B with product
Watanabe, M., Kamachi, S., Mine, S.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Exo-beta-D-glucosaminidase | 786 | Thermococcus kodakarensis KOD1 | Mutation(s): 0 Gene Names: Tk-Glm, TK1754 | 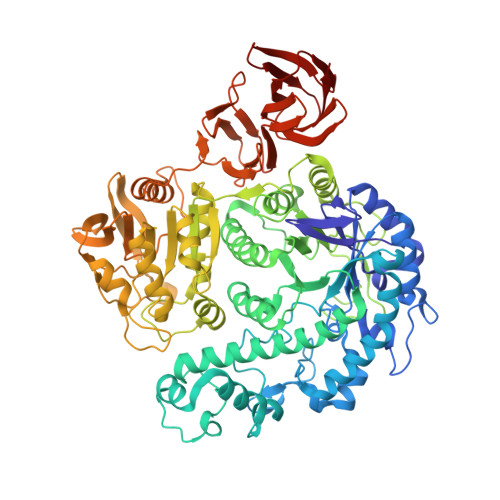 | |
UniProt | |||||
Find proteins for Q76HN4 (Thermococcus kodakarensis (strain ATCC BAA-918 / JCM 12380 / KOD1)) Explore Q76HN4 Go to UniProtKB: Q76HN4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q76HN4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| B3P Query on B3P | M [auth A] | 2-[3-(2-HYDROXY-1,1-DIHYDROXYMETHYL-ETHYLAMINO)-PROPYLAMINO]-2-HYDROXYMETHYL-PROPANE-1,3-DIOL C11 H26 N2 O6 HHKZCCWKTZRCCL-UHFFFAOYSA-N |  | ||
| GCS Query on GCS | C [auth A], N [auth B] | 2-amino-2-deoxy-beta-D-glucopyranose C6 H13 N O5 MSWZFWKMSRAUBD-QZABAPFNSA-N |  | ||
| PEG Query on PEG | D [auth A] E [auth A] F [auth A] G [auth A] H [auth A] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| PPI Query on PPI | J [auth A] K [auth A] L [auth A] U [auth B] V [auth B] | PROPANOIC ACID C3 H6 O2 XBDQKXXYIPTUBI-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 76.585 | α = 90 |
| b = 119.991 | β = 98.66 |
| c = 85.902 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| DENZO | data reduction |
| SCALEPACK | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| The Japan Society for the Promotion of Sciences | Japan | 25450143 |