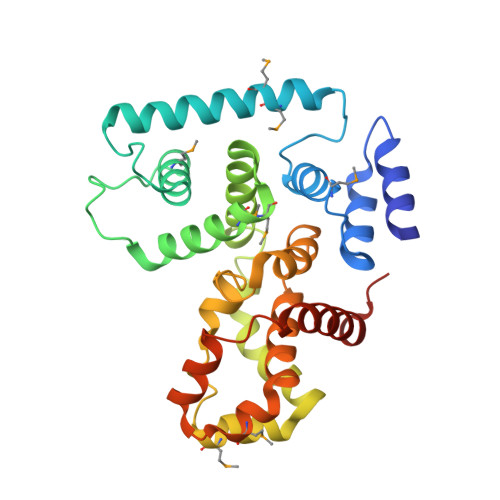
Structural Basis of the Interaction between Tuberous Sclerosis Complex 1 (TSC1) and Tre2-Bub2-Cdc16 Domain Family Member 7 (TBC1D7).
Qin, J., Wang, Z., Hoogeveen-Westerveld, M., Shen, G., Gong, W., Nellist, M., Xu, W.(2016) J Biol Chem 291: 8591-8601
- PubMed: 26893383
- DOI: https://doi.org/10.1074/jbc.M115.701870
- Primary Citation of Related Structures:
5EJC - PubMed Abstract:
Mutations in TSC1 or TSC2 cause tuberous sclerosis complex (TSC), an autosomal dominant disorder characterized by the occurrence of benign tumors in various vital organs and tissues. TSC1 and TSC2, the TSC1 and TSC2 gene products, form the TSC protein complex that senses specific cellular growth conditions to control mTORC1 signaling. TBC1D7 is the third subunit of the TSC complex, and helps to stabilize the TSC1-TSC2 complex through its direct interaction with TSC1. Homozygous inactivation of TBC1D7 causes intellectual disability and megaencephaly. Here we report the crystal structure of a TSC1-TBC1D7 complex and biochemical characterization of the TSC1-TBC1D7 interaction. TBC1D7 interacts with the C-terminal region of the predicted coiled-coil domain of TSC1. The TSC1-TBC1D7 interface is largely hydrophobic, involving the α4 helix of TBC1D7. Each TBC1D7 molecule interacts simultaneously with two parallel TSC1 helices from two TSC1 molecules, suggesting that TBC1D7 may stabilize the TSC complex by tethering the C-terminal ends of two TSC1 coiled-coils.
Organizational Affiliation:
From the Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China, the Department of Biological Structure, University of Washington, Seattle, Washington 98195, the University of Chinese Academy of Sciences, Beijing 100049, China.