Structure-Activity Studies of beta-Hairpin Peptide Inhibitors of the Plasmodium falciparum AMA1-RON2 Interaction.
Wang, G., Drinkwater, N., Drew, D.R., MacRaild, C.A., Chalmers, D.K., Mohanty, B., Lim, S.S., Anders, R.F., Beeson, J.G., Thompson, P.E., McGowan, S., Simpson, J.S., Norton, R.S., Scanlon, M.J.(2016) J Mol Biol 428: 3986-3998
- PubMed: 27422009
- DOI: https://doi.org/10.1016/j.jmb.2016.07.001
- Primary Citation of Related Structures:
4Z09, 4Z0D, 4Z0E, 4Z0F - PubMed Abstract:
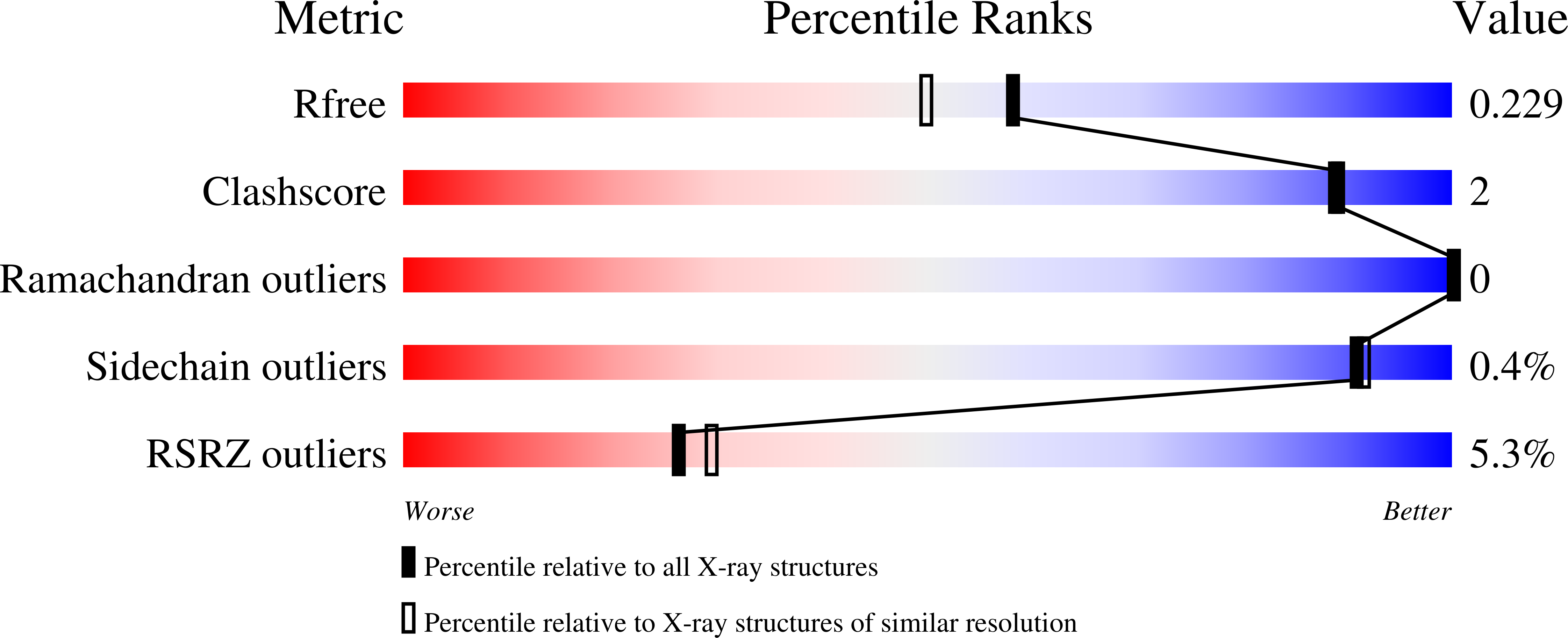
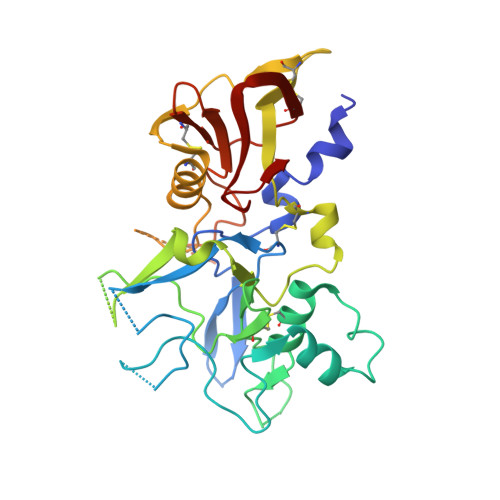
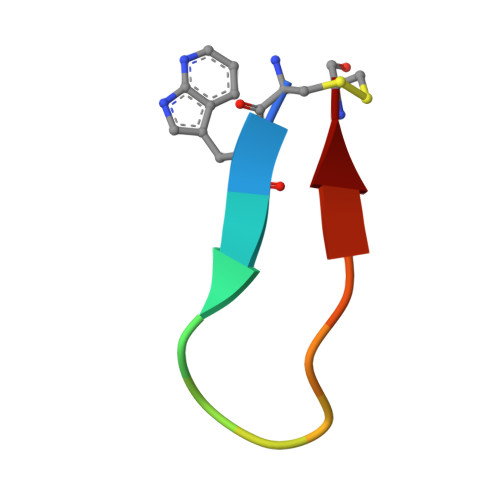
The interaction between apical membrane antigen 1 (AMA1) and rhoptry neck protein 2 (RON2) plays a key role in the invasion of red blood cells by Plasmodium parasites. Disruption of this critical protein-protein interaction represents a promising avenue for antimalarial drug discovery. In this work, we exploited a 13-residue β-hairpin based on the C-terminal loop of RON2 to probe a conserved binding site on Plasmodium falciparum AMA1. A series of mutations was synthetically engineered into β-hairpin peptides to establish structure-activity relationships. The best mutations improved the binding affinity of the β-hairpin peptide by ~7-fold for 3D7 AMA1 and ~14-fold for FVO AMA1. We determined the crystal structures of several β-hairpin peptides in complex with AMA1 in order to define the structural features and specific interactions that contribute to improved binding affinity. The same mutations in the longer RON2sp2 peptide (residues 2027-2055 of RON2) increased the binding affinity by >30-fold for 3D7 and FVO AMA1, producing K D values of 2.1nM and 0.4nM, respectively. To our knowledge, this is the most potent strain-transcending peptide reported to date and represents a valuable tool to characterize the AMA1-RON2 interaction.
Organizational Affiliation:
Medicinal Chemistry, Monash Institute of Pharmaceutical Sciences, Monash University, 381 Royal Parade, Parkville, Victoria 3052, Australia.