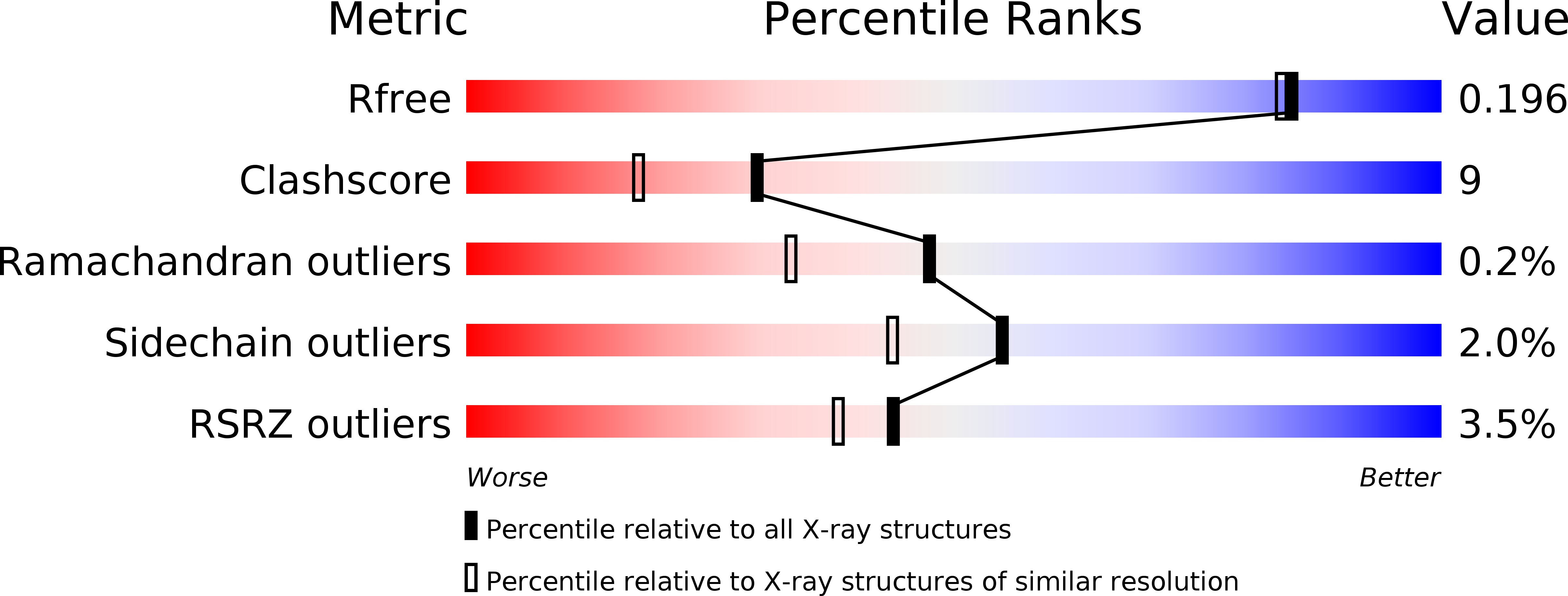
Acyl-CoA Dehydrogenase Drives Heat Adaptation by Sequestering Fatty Acids
Ma, D.K., Li, Z., Lu, A.Y., Sun, F., Chen, S., Rothe, M., Menzel, R., Sun, F., Horvitz, H.R.(2015) Cell 161: 1152-1163
- PubMed: 25981666
- DOI: https://doi.org/10.1016/j.cell.2015.04.026
- Primary Citation of Related Structures:
4Y9J, 4Y9L - PubMed Abstract:
Cells adapt to temperature shifts by adjusting levels of lipid desaturation and membrane fluidity. This fundamental process occurs in nearly all forms of life, but its mechanism in eukaryotes is unknown. We discovered that the evolutionarily conserved Caenorhabditis elegans gene acdh-11 (acyl-CoA dehydrogenase [ACDH]) facilitates heat adaptation by regulating the lipid desaturase FAT-7. Human ACDH deficiency causes the most common inherited disorders of fatty acid oxidation, with syndromes that are exacerbated by hyperthermia. Heat upregulates acdh-11 expression to decrease fat-7 expression. We solved the high-resolution crystal structure of ACDH-11 and established the molecular basis of its selective and high-affinity binding to C11/C12-chain fatty acids. ACDH-11 sequesters C11/C12-chain fatty acids and prevents these fatty acids from activating nuclear hormone receptors and driving fat-7 expression. Thus, the ACDH-11 pathway drives heat adaptation by linking temperature shifts to regulation of lipid desaturase levels and membrane fluidity via an unprecedented mode of fatty acid signaling.
Organizational Affiliation:
Department of Biology, Howard Hughes Medical Institute, McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. Electronic address: dengke.ma@ucsf.edu.