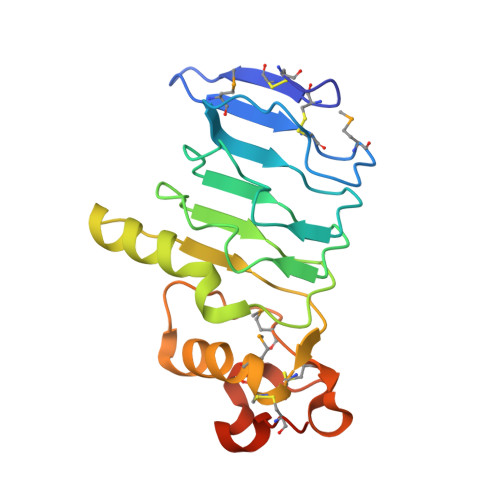
Structure of a variable lymphocyte receptor-like protein from the amphioxus Branchiostoma floridae.
Cao, D.D., Liao, X., Cheng, W., Jiang, Y.L., Wang, W.J., Li, Q., Chen, J.Y., Chen, Y., Zhou, C.Z.(2016) Sci Rep 6: 19951-19951
- PubMed: 26821753
- DOI: https://doi.org/10.1038/srep19951
- Primary Citation of Related Structures:
4XSQ - PubMed Abstract:
Discovery of variable lymphocyte receptors (VLRs) in agnathans (jawless fish) has brought the origin of adaptive immunity system (AIS) forward to 500 million years ago accompanying with the emergence of vertebrates. Previous findings indicated that amphioxus, a representative model organism of chordate, also possesses some homologs of the basic components of TCR/BCR-based AIS, but it remains unknown if there exist any components of VLR-based AIS in amphioxus. Bioinformatics analyses revealed the amphioxus Branchiostoma floridae encodes a group of putative VLR-like proteins. Here we reported the 1.79 Å crystal structure of Bf66946, which forms a crescent-shaped structure of five leucine-rich repeats (LRRs). Structural comparisons indicated that Bf66946 resembles the lamprey VLRC. Further electrostatic potential analyses showed a negatively-charged patch at the concave of LRR solenoid structure that might be responsible for antigen recognition. Site-directed mutagenesis combined with bacterial binding assays revealed that Bf66946 binds to the surface of Gram-positive bacteria Staphylococcus aureus and Streptococcus pneumonia via a couple of acidic residues at the concave. In addition, the closest homolog of Bf66946 is highly expressed in the potential immune organ gill of Branchiostoma belcheri. Altogether, our findings provide the first structural evidence for the emergence of VLR-like molecules in the basal chordates.
Organizational Affiliation:
Hefei National Laboratory for Physical Sciences at the Microscale and School of Life Sciences, University of Science and Technology of China, Hefei Anhui 230027, China.