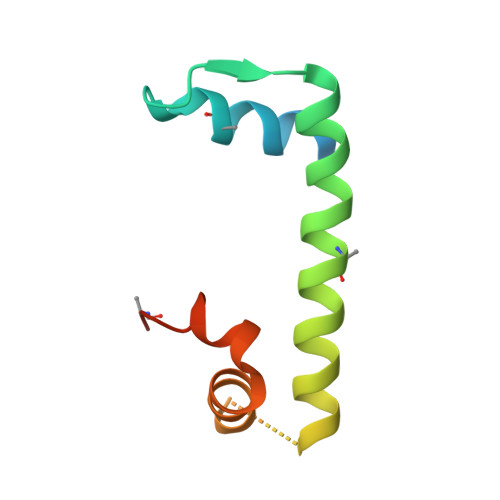
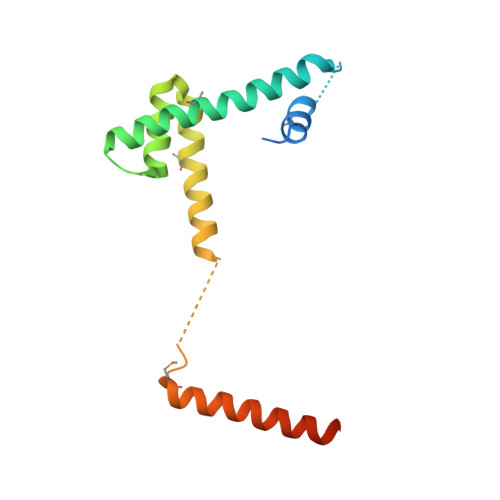
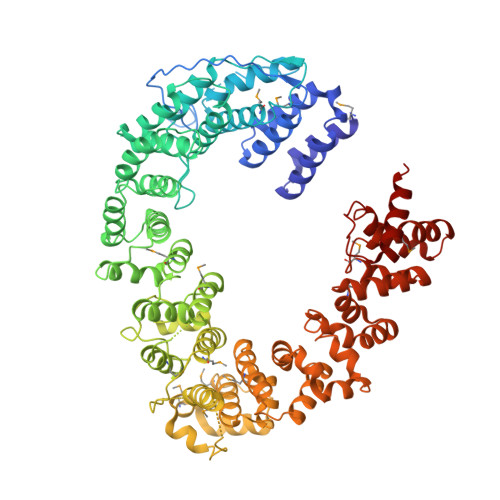
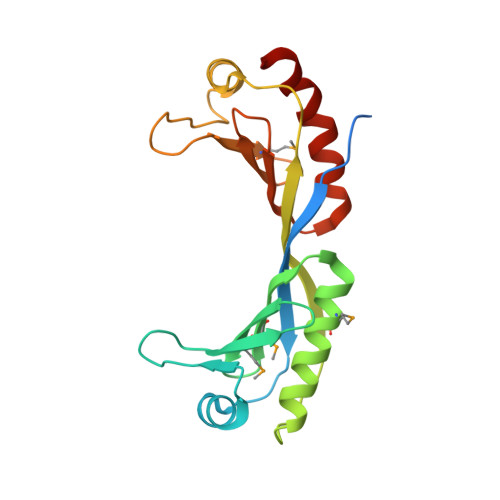
Structural basis for recognition and remodeling of the TBP:DNA:NC2 complex by Mot1.
Butryn, A., Schuller, J.M., Stoehr, G., Runge-Wollmann, P., Forster, F., Auble, D.T., Hopfner, K.P.(2015) Elife 4
- PubMed: 26258880
- DOI: https://doi.org/10.7554/eLife.07432
- Primary Citation of Related Structures:
4WZS - PubMed Abstract:
Swi2/Snf2 ATPases remodel substrates such as nucleosomes and transcription complexes to control a wide range of DNA-associated processes, but detailed structural information on the ATP-dependent remodeling reactions is largely absent. The single subunit remodeler Mot1 (modifier of transcription 1) dissociates TATA box-binding protein (TBP):DNA complexes, offering a useful system to address the structural mechanisms of Swi2/Snf2 ATPases. Here, we report the crystal structure of the N-terminal domain of Mot1 in complex with TBP, DNA, and the transcription regulator negative cofactor 2 (NC2). Our data show that Mot1 reduces DNA:NC2 interactions and unbends DNA as compared to the TBP:DNA:NC2 state, suggesting that Mot1 primes TBP:NC2 displacement in an ATP-independent manner. Electron microscopy and cross-linking data suggest that the Swi2/Snf2 domain of Mot1 associates with the upstream DNA and the histone fold of NC2, thereby revealing parallels to some nucleosome remodelers. This study provides a structural framework for how a Swi2/Snf2 ATPase interacts with its substrate DNA:protein complex.
Organizational Affiliation:
Gene Center, Department of Biochemistry, Ludwig Maximilian University, Munich, Germany.