Structures of designed armadillo-repeat proteins show propagation of inter-repeat interface effects.
Reichen, C., Madhurantakam, C., Hansen, S., Grutter, M.G., Pluckthun, A., Mittl, P.R.(2016) Acta Crystallogr D Struct Biol 72: 168-175
- PubMed: 26894544
- DOI: https://doi.org/10.1107/S2059798315023116
- Primary Citation of Related Structures:
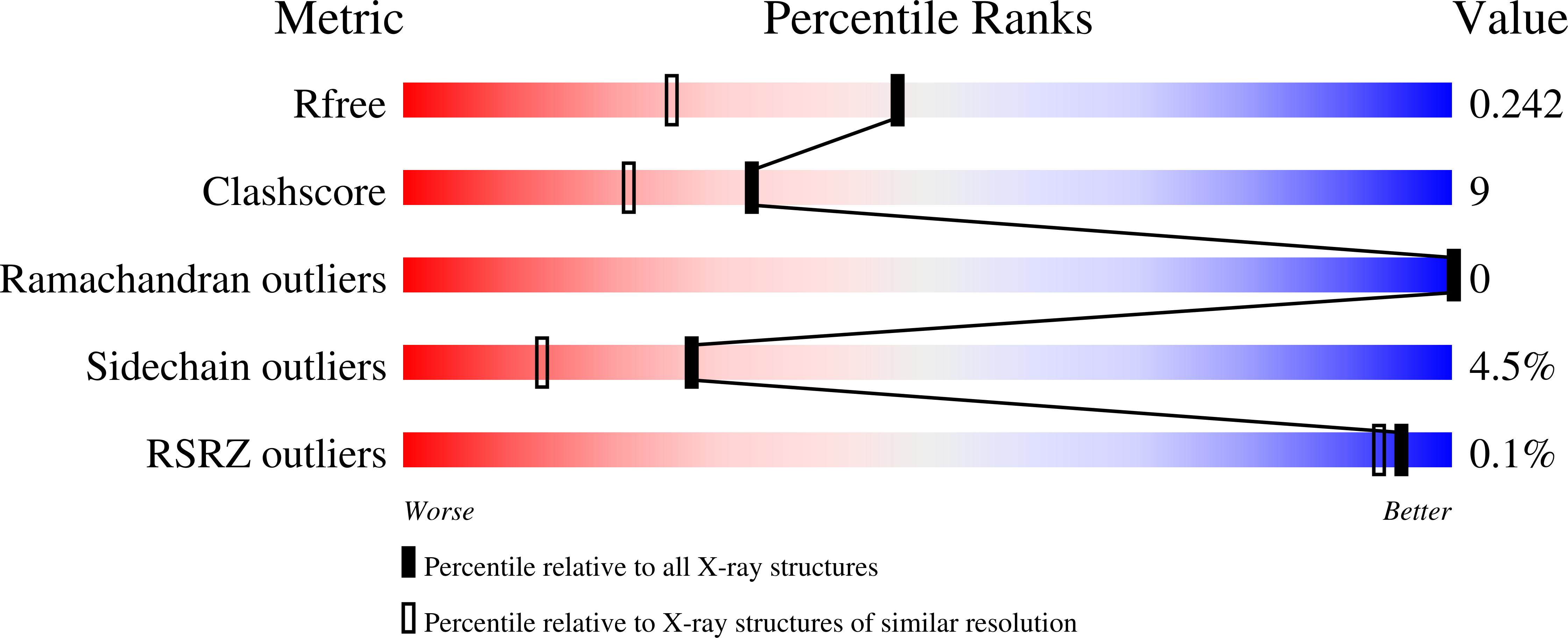
4V3O, 4V3Q, 4V3R - PubMed Abstract:
The armadillo repeat serves as a scaffold for the development of modular peptide-recognition modules. In order to develop such a system, three crystal structures of designed armadillo-repeat proteins with third-generation N-caps (YIII-type), four or five internal repeats (M-type) and second-generation C-caps (AII-type) were determined at 1.8 Å (His-YIIIM4AII), 2.0 Å (His-YIIIM5AII) and 1.95 Å (YIIIM5AII) resolution and compared with those of variants with third-generation C-caps. All constructs are full consensus designs in which the internal repeats have exactly the same sequence, and hence identical conformations of the internal repeats are expected. The N-cap and internal repeats M1 to M3 are indeed extremely similar, but the comparison reveals structural differences in internal repeats M4 and M5 and the C-cap. These differences are caused by long-range effects of the C-cap, contacting molecules in the crystal, and the intrinsic design of the repeat. Unfortunately, the rigid-body movement of the C-terminal part impairs the regular arrangement of internal repeats that forms the putative peptide-binding site. The second-generation C-cap improves the packing of buried residues and thereby the stability of the protein. These considerations are useful for future improvements of an armadillo-repeat-based peptide-recognition system.
Organizational Affiliation:
Department of Biochemistry, University of Zürich, Winterthurerstrasse 190, 8057 Zürich, Switzerland.