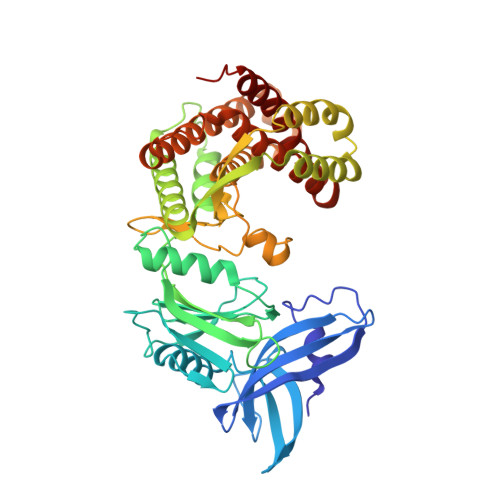
Structure of mycobacterial maltokinase, the missing link in the essential GlgE-pathway.
Fraga, J., Maranha, A., Mendes, V., Pereira, P.J., Empadinhas, N., Macedo-Ribeiro, S.(2015) Sci Rep 5: 8026-8026
- PubMed: 25619172
- DOI: https://doi.org/10.1038/srep08026
- Primary Citation of Related Structures:
4U94, 4U98, 4WZY - PubMed Abstract:
A novel four-step pathway identified recently in mycobacteria channels trehalose to glycogen synthesis and is also likely involved in the biosynthesis of two other crucial polymers: intracellular methylglucose lipopolysaccharides and exposed capsular glucan. The structures of three of the intervening enzymes - GlgB, GlgE, and TreS - were recently reported, providing the first templates for rational drug design. Here we describe the structural characterization of the fourth enzyme of the pathway, mycobacterial maltokinase (Mak), uncovering a eukaryotic-like kinase (ELK) fold, similar to methylthioribose kinases and aminoglycoside phosphotransferases. The 1.15 Å structure of Mak in complex with a non-hydrolysable ATP analog reveals subtle structural rearrangements upon nucleotide binding in the cleft between the N- and the C-terminal lobes. Remarkably, this new family of ELKs has a novel N-terminal domain topologically resembling the cystatin family of protease inhibitors. By interfacing with and restraining the mobility of the phosphate-binding region of the N-terminal lobe, Mak's unusual N-terminal domain might regulate its phosphotransfer activity and represents the most likely anchoring point for TreS, the upstream enzyme in the pathway. By completing the gallery of atomic-detail models of an essential pathway, this structure opens new avenues for the rational design of alternative anti-tubercular compounds.
Organizational Affiliation:
IBMC - Instituto de Biologia Molecular e Celular, Universidade do Porto, Porto, Portugal.