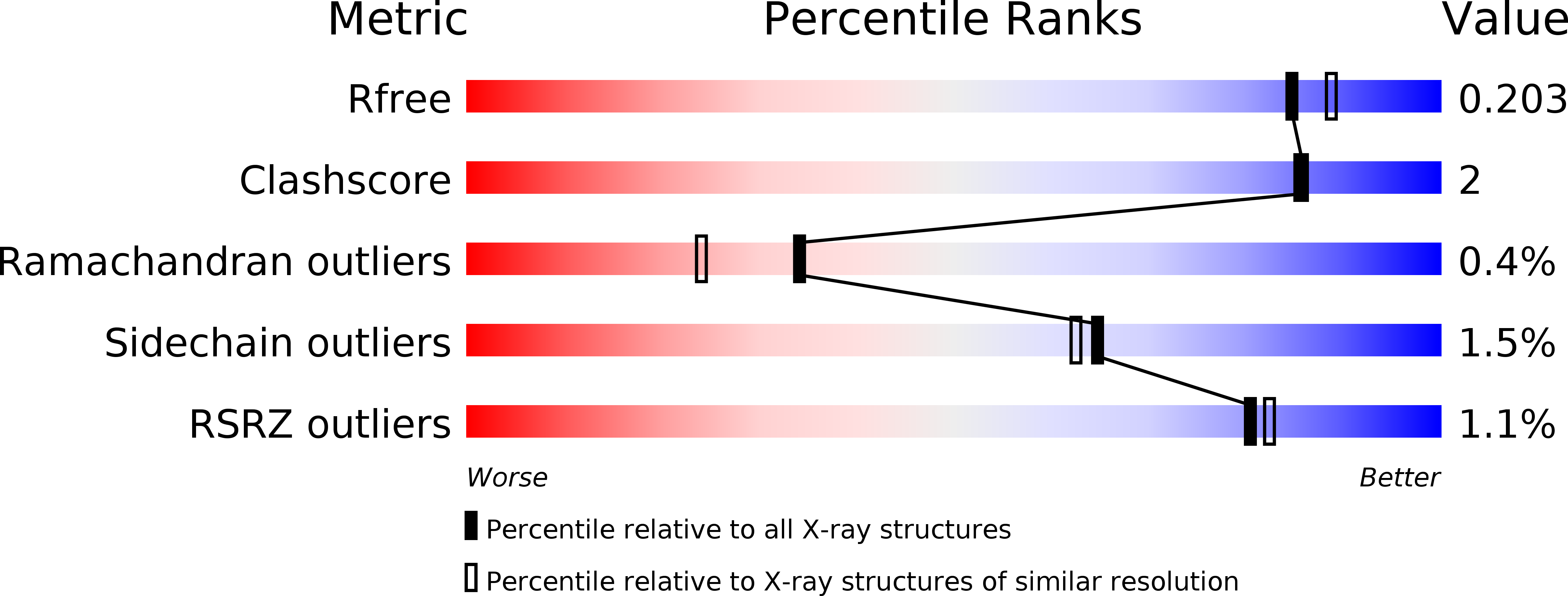
Crystal Structure of LacI Transcriptional Regulator Rbsr from Enterococcus faecium, Target EFI-512930
Patskovsky, Y., Toro, R., Bhosle, R., Al Obaidi, N., Chamala, S., Scott Glenn, A., Attonito, J.D., Chowdhury, S., Lafleur, J., Siedel, R.D., Hillerich, B., Love, J., Whalen, K.L., Gerlt, J.A., Almo, S.C.To be published.