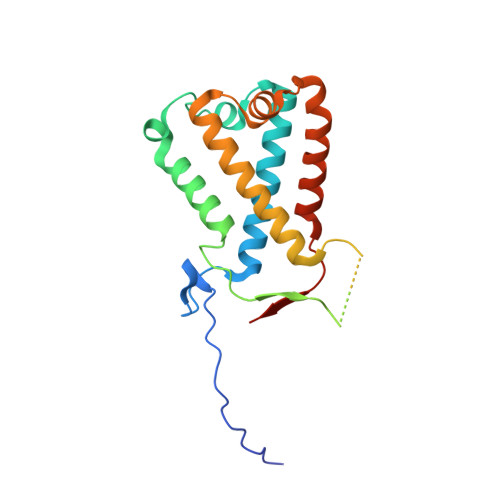
Crystal structures of the PsbS protein essential for photoprotection in plants.
Fan, M., Li, M., Liu, Z., Cao, P., Pan, X., Zhang, H., Zhao, X., Zhang, J., Chang, W.(2015) Nat Struct Mol Biol 22: 729-735
- PubMed: 26258636
- DOI: https://doi.org/10.1038/nsmb.3068
- Primary Citation of Related Structures:
4RI2, 4RI3 - PubMed Abstract:
The photosystem II protein PsbS has an essential role in qE-type nonphotochemical quenching, which protects plants from photodamage under excess light conditions. qE is initiated by activation of PsbS by low pH, but the mechanism of PsbS action remains elusive. Here we report the low-pH crystal structures of PsbS from spinach in its free form and in complex with the qE inhibitor N,N'-dicyclohexylcarbodiimide (DCCD), revealing that PsbS adopts a unique folding pattern, and, unlike other members of the light-harvesting-complex superfamily, it is a noncanonical pigment-binding protein. Structural and biochemical evidence shows that both active and inactive PsbS form homodimers in the thylakoid membranes, and DCCD binding disrupts the lumenal intermolecular hydrogen bonds of the active PsbS dimer. Activation of PsbS by low pH during qE may involve a conformational change associated with altered lumenal intermolecular interactions of the PsbS dimer.
Organizational Affiliation:
National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China.