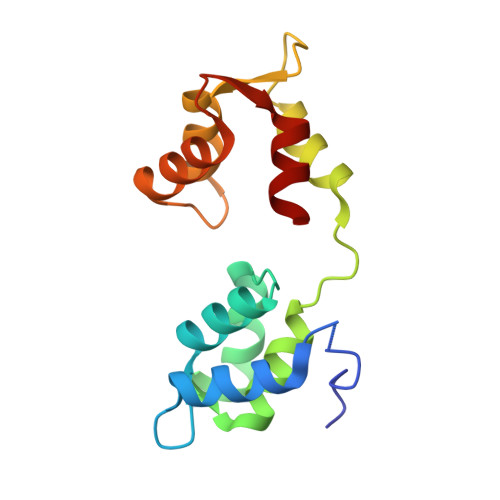
Targeting a Dynamic Protein-Protein Interaction: Fragment Screening against the Malaria Myosin A Motor Complex.
Douse, C.H., Vrielink, N., Wenlin, Z., Cota, E., Tate, E.W.(2015) ChemMedChem 10: 134-143
- PubMed: 25367834
- DOI: https://doi.org/10.1002/cmdc.201402357
- Primary Citation of Related Structures:
4R1E - PubMed Abstract:
Motility is a vital feature of the complex life cycle of Plasmodium falciparum, the apicomplexan parasite that causes human malaria. Processes such as host cell invasion are thought to be powered by a conserved actomyosin motor (containing myosin A or myoA), correct localization of which is dependent on a tight interaction with myosin A tail domain interacting protein (MTIP) at the inner membrane of the parasite. Although disruption of this protein-protein interaction represents an attractive means to investigate the putative roles of myoA-based motility and to inhibit the parasitic life cycle, no small molecules have been identified that bind to MTIP. Furthermore, it has not been possible to obtain a crystal structure of the free protein, which is highly dynamic and unstable in the absence of its natural myoA tail partner. Herein we report the de novo identification of the first molecules that bind to and stabilize MTIP via a fragment-based, integrated biophysical approach and structural investigations to examine the binding modes of hit compounds. The challenges of targeting such a dynamic system with traditional fragment screening workflows are addressed throughout.
Organizational Affiliation:
Department of Chemistry, Imperial College London, South Kensington, London SW7 2AZ (UK); Centre for Structural Biology, Department of Life Sciences, Imperial College London, South Kensington, London SW7 2AZ (UK); Institute of Chemical Biology, Imperial College London, South Kensington, London SW7 2AZ (UK). christopher.douse@googlemail.com.