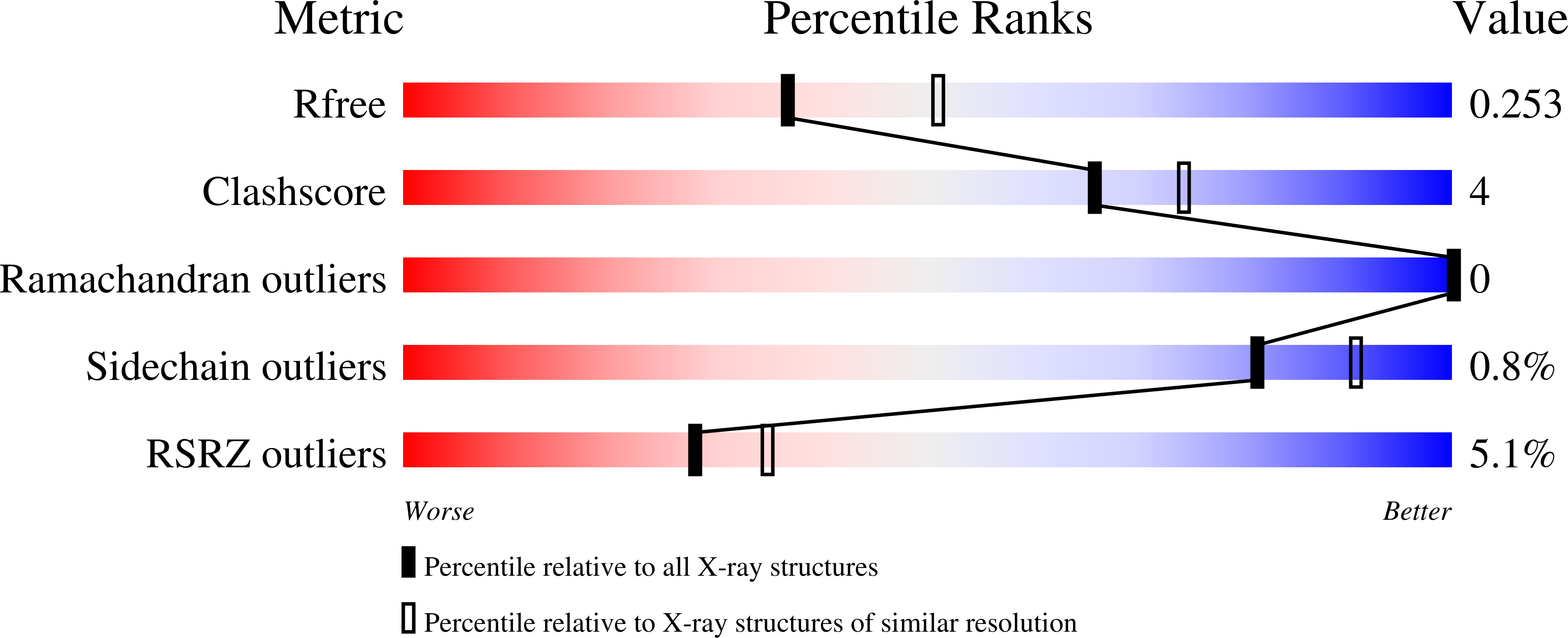
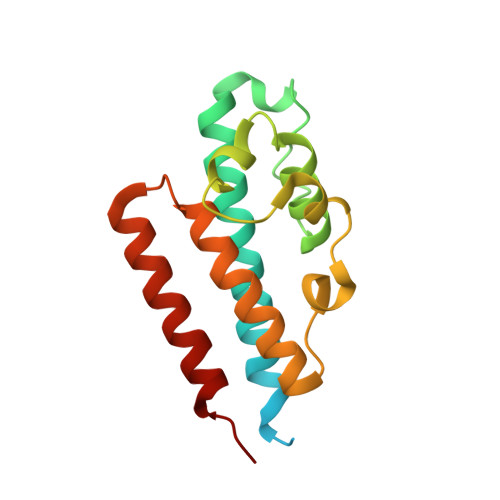
High-resolution crystal structure reveals a HEPN domain at the C-terminal region of S. cerevisiae RNA endonuclease Swt1.
Peng, S., Zhou, K., Wang, W., Gao, Z., Dong, Y., Liu, Q.(2014) Biochem Biophys Res Commun 453: 826-832
- PubMed: 25450355
- DOI: https://doi.org/10.1016/j.bbrc.2014.10.040
- Primary Citation of Related Structures:
4PQZ - PubMed Abstract:
Swt1 is an RNA endonuclease that plays an important role in quality control of nuclear messenger ribonucleoprotein particles (mRNPs) in eukaryotes; however, its structural details remain to be elucidated. Here, we report the crystal structure of the C-terminal (CT) domain of Swt1 from Saccharomyces cerevisiae, which shares common characteristics of higher eukaryotes and prokaryotes nucleotide binding (HEPN) domain superfamily. To study in detail the full-length protein structure, we analyzed the low-resolution architecture of Swt1 in solution using small angle X-ray scattering (SAXS) method. Both the CT domain and middle domain exhibited a good fit upon superimposing onto the molecular envelope of Swt1. Our study provides the necessary structural information for detailed analysis of the functional role of Swt1, and its importance in the process of nuclear mRNP surveillance.
Organizational Affiliation:
Multidisciplinary Research Center, Institute for High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China. Electronic address: pengsx@ihep.ac.cn.