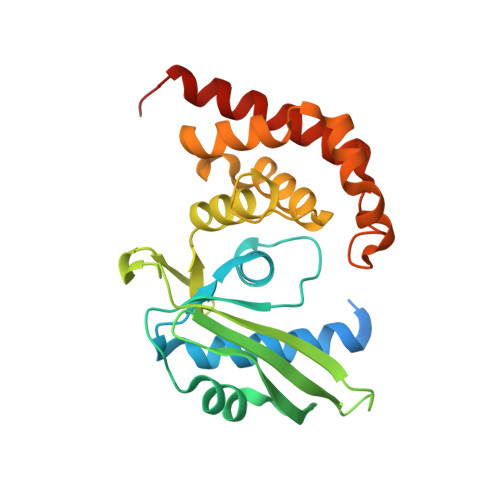
Crystal structure of JHP933 from Helicobacter pylori J99 shows two-domain architecture with a DUF1814 family nucleotidyltransferase domain and a helical bundle domain.
Yoon, J.Y., Lee, S.J., Kim, D.J., Lee, B.J., Yang, J.K., Suh, S.W.(2014) Proteins 82: 2275-2281
- PubMed: 24677396
- DOI: https://doi.org/10.1002/prot.24572
- Primary Citation of Related Structures:
4OK0 - PubMed Abstract:
The jhp0933 gene in the plasticity region of Helicobacter pylori J99 encodes a hypothetical protein (JHP933), which may play some roles in pathogenesis. Here, we have determined the crystal structure of JHP933 at 2.17 Å. It represents the first crystal structure of the DUF1814 protein family. JHP933 consists of two domains: an N-terminal domain of the nucleotidyltransferase (NTase) fold and a C-terminal helix bundle domain. A highly positively charged surface patch exists adjacent to the putative NTP binding site. Structural similarity of JHP933 to known NTases is very remote, suggesting that it may function as a novel NTase.
Organizational Affiliation:
Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul, 151-742, Republic of Korea.