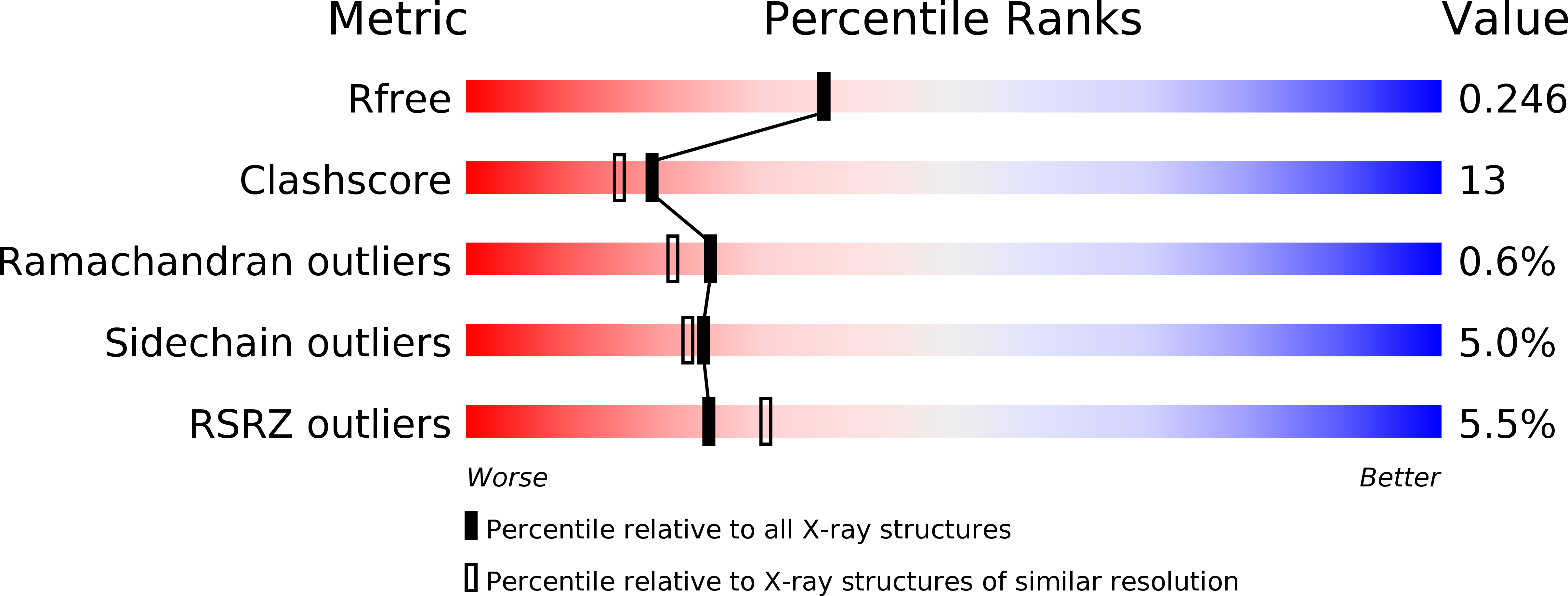
The crystal structure of zebrafish IL-22 reveals an evolutionary, conserved structure highly similar to that of human IL-22.
Siupka, P., Hamming, O.J., Fretaud, M., Luftalla, G., Levraud, J.P., Hartmann, R.(2014) Genes Immun 15: 293-302
- PubMed: 24833303
- DOI: https://doi.org/10.1038/gene.2014.18
- Primary Citation of Related Structures:
4O6K - PubMed Abstract:
The class II cytokine family consists of small α-helical signaling proteins including the interleukin-10 (IL-10)/IL-22 family, as well as interferons (IFNs). They regulate the innate immune response and in addition have an important role in protecting epithelial tissues. Teleost fish possess a class II cytokine system surprisingly similar to that of humans, and thus zebrafish offers an attractive model organism for investigating the role of class II cytokines in inflammation. However, the evolution of class II cytokines is critical to understand if we are to take full advantage of zebrafish as a model system. The small size and fast evolution of these cytokines obscure phylogenetic analyses based purely on sequences, but one can overcome this obstacle by using information contained within the structure of those molecules. Here we present the crystal structure of IL-22 from zebrafish (zIL-22) solved at 2.1 Å, which displays a typical class II cytokine architecture. We generated a structure-guided alignment of vertebrate class II cytokines and used it for phylogenetic analysis. Our analysis suggests that IL-22 and IL-26 arose early during the evolution of the IL-10-like cytokines. Thus, we propose an evolutionary scenario of class II cytokines in vertebrates, based on genomic and structural data.
Organizational Affiliation:
Department of Molecular Biology and Genetics, Aarhus University, Aarhus, Denmark.