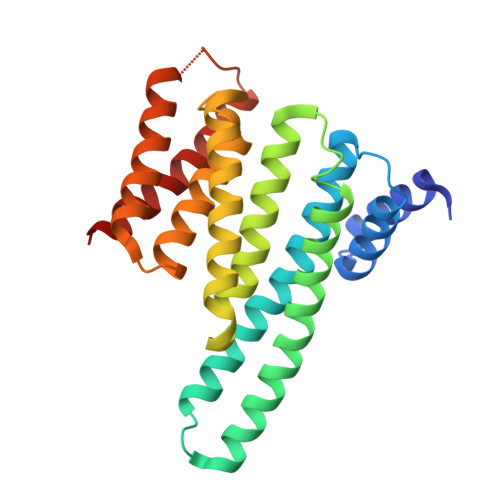
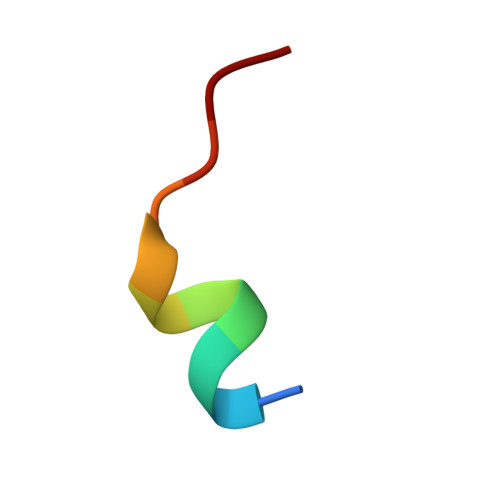
Constrained peptides with target-adapted cross-links as inhibitors of a pathogenic protein-protein interaction.
Glas, A., Bier, D., Hahne, G., Rademacher, C., Ottmann, C., Grossmann, T.N.(2014) Angew Chem Int Ed Engl 53: 2489-2493
- PubMed: 24504455
- DOI: https://doi.org/10.1002/anie.201310082
- Primary Citation of Related Structures:
4N7G, 4N7Y, 4N84 - PubMed Abstract:
Bioactive conformations of peptides can be stabilized by macrocyclization, resulting in increased target affinity and activity. Such macrocyclic peptides proved useful as modulators of biological functions, in particular as inhibitors of protein-protein interactions (PPI). However, most peptide-derived PPI inhibitors involve stabilized α-helices, leaving a large number of secondary structures unaddressed. Herein, we present a rational approach towards stabilization of an irregular peptide structure, using hydrophobic cross-links that replace residues crucially involved in target binding. The molecular basis of this interaction was elucidated by X-ray crystallography and isothermal titration calorimetry. The resulting cross-linked peptides inhibit the interaction between human adaptor protein 14-3-3 and virulence factor exoenzyme S. Taking into consideration that irregular peptide structures participate widely in PPIs, this approach provides access to novel peptide-derived inhibitors.
Organizational Affiliation:
Chemical Genomics Centre of the Max Planck Society, Otto-Hahn-Strasse 15, 44227 Dortmund (Germany).