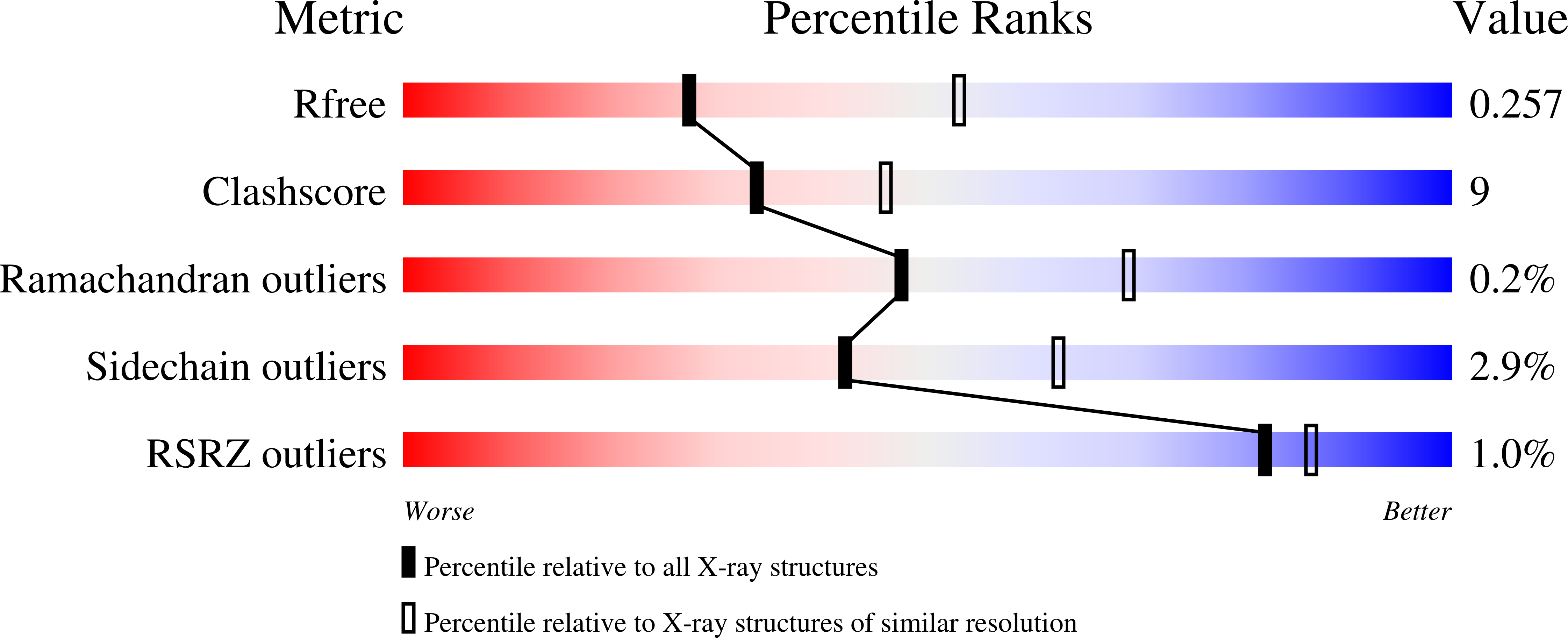
Molecular Structure of the Brucella abortus Metalloprotein RicA, a Rab2-Binding Virulence Effector.
Herrou, J., Crosson, S.(2013) Biochemistry 52: 9020-9028
- PubMed: 24251537
- DOI: https://doi.org/10.1021/bi401373r
- Primary Citation of Related Structures:
4N27 - PubMed Abstract:
The Gram-negative intracellular pathogen Brucella abortus is the causative agent of brucellosis, which is among the most common zoonoses globally. The B. abortus RicA protein binds the host-expressed guanosine nucleotide-binding protein, Rab2, and modulates B. abortus infection biology. We have solved the first X-ray crystal structure of RicA to 2.7 Å resolution and have quantified the affinity of RicA binding to human Rab2 in its GDP-bound and nucleotide-free forms. RicA adopts a classic γ-carbonic anhydrase (γ-CA) fold containing a left-handed β-helix followed by a C-terminal α-helix. Two homotrimers of RicA occupy the crystallographic asymmetric unit. Though no zinc was included in the purification or crystallization buffers, zinc is contained within the RicA crystals, as demonstrated by X-ray fluorescence spectroscopy. Electron density for a Zn(2+) ion coordinated by three histidine residues is evident in the putative active site of RicA. However, purified RicA preparations do not exhibit carbonic anhydrase activity, suggesting that Zn(2+) may not be the physiologically relevant metal cofactor or that RicA is not a bona fide carbonic anhydrase enzyme. Isothermal titration calorimetry (ITC) measurements of purified RicA binding to purified human Rab2 and GDP-Rab2 revealed similar equilibrium affinities (Kd ≈ 35 and 40 μM, respectively). This study thus defines RicA as a Zn(2+)-binding γ-carbonic anhydrase-like protein that binds the human membrane fusion/trafficking protein Rab2 with low micromolar affinity in vitro. These results support a model in which γ-CA family proteins may evolve unique cellular functions while retaining many of the structural hallmarks of archetypal γ-CA enzymes.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of Chicago , Chicago, Illinois 60637, United States.