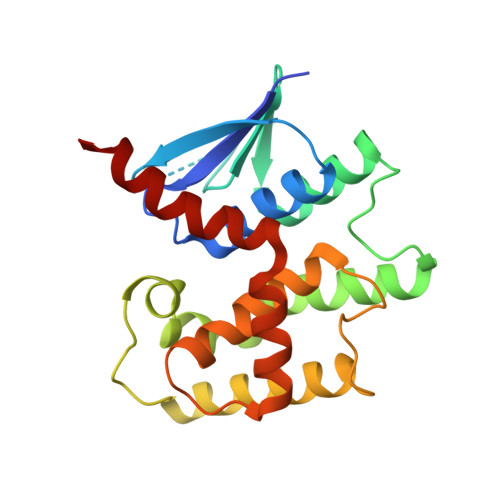
Crystal structure of a glutathione transferase family member from Acinetobacter baumannii, Target EFI-501785, apo structure
Vetting, M.W., Toro, R., Bhosle, R., Al Obaidi, N.F., Morisco, L.L., Wasserman, S.R., Sojitra, S., Stead, M., Scott Glenn, A., Chowdhury, S., Evans, B., Hillerich, B., Love, J., Seidel, R.D., Imker, H.J., Gerlt, J.A., Armstrong, R.N., Almo, S.C.To be published.